First-Ever Success in Accurate Determination of Radiation-Damage-Free Crystal Structure of Protein at SACLA Facility (Press Release)
- Release Date
- 12 May, 2014
- SACLA
RIKEN
University of Hyogo
Japan Synchrotron Radiation Research Institute (JASRI)
Osaka University
Okayama University
Key points
• High-resolution crystal structure determination of protein free from radiation damage
• Steady step toward motion capture of functioning protein
• Development of new method of linking life science and materials science
|
A joint research group of RIKEN (President, Ryoji Noyori), the University of Hyogo (President, Masayoshi Kiyohara), JASRI (President, Yoshiharu Doi), Osaka University (President, Toshio Hirano), and Okayama University (President, Kiyoshi Morita) has successfully developed a method of femtosecond crystallography using an X-ray laser at the SACLA[1] facility, an X-ray free-electron laser (XFEL) facility. This is a milestone that marks the beginning of a new era of X-ray laser crystallography and was established in the International Year of Crystallography (IYCr2014, designated by the United Nations), the 101st year after the world's first successful X-ray crystallography. The group was led by Kunio Hirata (senior technical scientist), Hideo Ago (senior research scientist), and Masaki Yamamoto (Group Director) of the Research Infrastructure Group, RIKEN SPring-8 Center (Director, Tetsuya Ishikawa), and Kyoko Shinzawa-Itoh (associate professor) and Shinya Yoshikawa (specially appointed professor) of the University of Hyogo. Publication: |
《Figure》
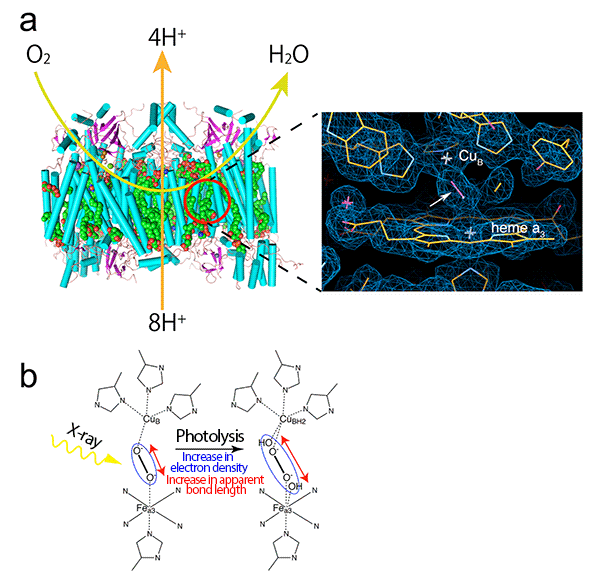
(a) Cytochrome c oxidase is a proton pump powered by the free energy derived from the reduction of oxygen from the air at the active site[6] (red circle), thus a concentration gradient of proton is formed across the biological membrane. The concentration gradient of proton is indispensable for the biosynthesis of adenosine triphosphate as the energy substance for life. The reduction of oxygen to water and the proton pumping across the mitochondrial membrane are schematically shown by the light green and orange arrows, respectively. The right image is a magnified view of the radiation-damage-free active site with electron density observed at SACLA in this research. As shown by the white arrow, a peroxide anion formed from oxygen binds to two metal ions (CuB and Fea3 of heme a3) that form the active site.
(b) In conventional X-ray crystallography, peroxide anions are broken by X-ray irradiation, resulting in an increase in the apparent bond length corresponding to the electron density of peroxide anions (shown in the blue ellipse). Thus, the structure of the active site before X-ray irradiation is not accurately determined by conventional X-ray crystallography.
crystallography and schematics of diffraction imaging
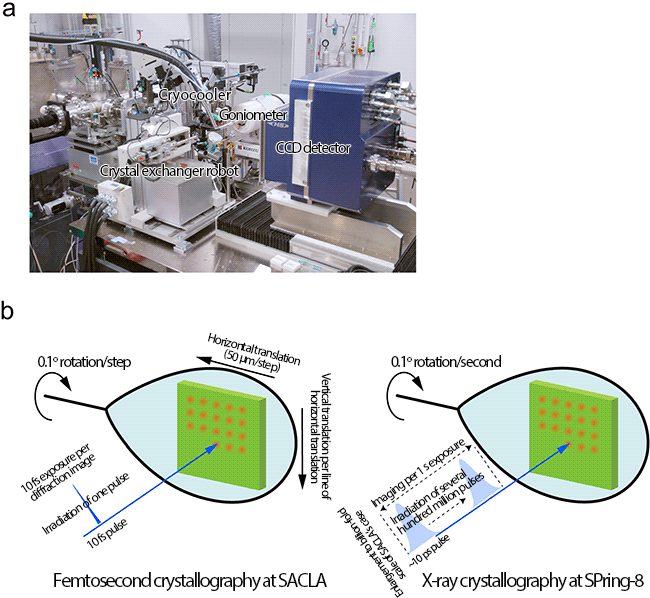
(a) Setup for taking X-ray diffraction images of radiation-damage-free protein crystals using X-ray laser at SACLA. The target position of a crystal is accurately irradiated with an X-ray laser.
(b) In the femtosecond crystallography, an X-ray diffraction image is obtained by irradiating a 10 fs X-ray laser pulse. To avoid the effect of radiation damage by the previous irradiations, the crystal is translated before every X-ray pulse irradiation to record X-ray diffraction images from a fresh portion of the crystal. The crystal is also rotated by a certain degree between X-ray irradiations to obtain three-dimensional X-ray diffraction images. Conventional X-ray crystallography requires a long exposure time (several seconds), causing proteins to undergo radiation damage.
in the radiation damage free analysis,
and the active site of cytochrome c oxidase free of radiation damage
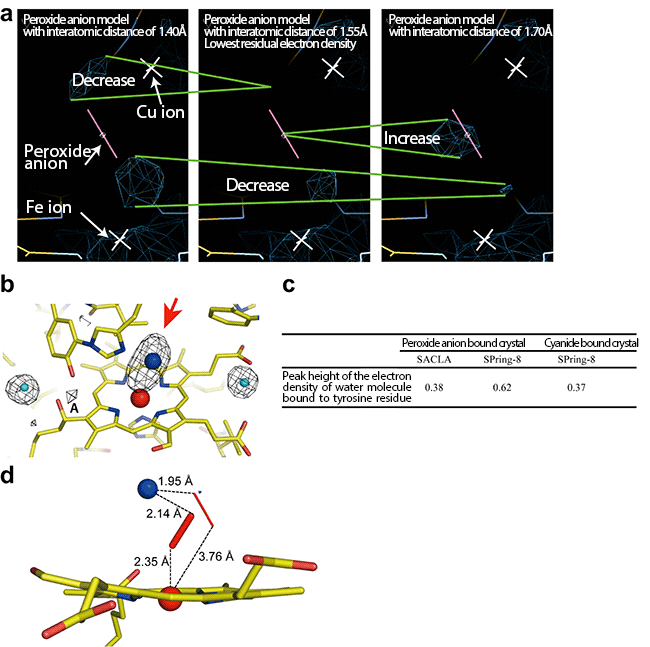
(a) To examine the interatomic distance between oxygen atoms of the peroxide anion that most clearly represents the electron density shown by the red arrow in (b), structure refinement calculations were carried out for peroxide anion models different in their interatomic distances. The residual electron density around the peroxide anion model (the navy-blue cage models) was lowest for a distance of 1.55 Å between oxygen atoms, meaning that the model with this interatomic distance is suitable. The changes in the residual electron density owing to the difference in the peroxide anion model used for calculation are shown by the two green lines.
(b) Radiation damage-free electron density map of oxygen-reducing site. The red arrow indicates the electron density of the peroxide anion that binds to two metal ions (red sphere, Fe ion; blue sphere, Cu ion). The almost comparable peak heights of the electron densities at position A of the peroxide-anion-bound and the cyanide-bound crystals clearly showed that the data collected by the femtosecond crystallography is free of radiation damage. The actual peak heights are shown in panel c. Water molecules generated as a result of radiation damage of peroxide are trapped by adjacent tyrosine residues (electron density shown by A). The cyanide does not form water molecules upon X-ray irradiation, therefore, the peak height of the peroxide-bound crystal comparable to the cyanide-bound form means that radiation damage of the peroxide molecule did not occur during the data collection. The light blue spheres indicate the water molecules. Yellow bars indicate the bonds between carbon atoms. Yellow bars with blue bars indicate the bonds between nitrogen and carbon atoms and those with red bars indicate the bonds between oxygen and carbon atoms.
(c) Comparison of peak heights of the electron density A in (b) among the data of the peroxide-bound cytochrome c oxidase collected at SACLA (the femtosecond crystallography) and at SPring-8[5] (the conventional crystallography) and the data of the cyanide-bound cytochrome c oxidase. The peak heights of the electron densities corresponding to A were normalized using the electron density around the water molecules, which remained unchanged upon X-ray irradiation.
(d) Radiation-damage-free structure of cytochrome c oxidase active site, corresponding to the site from Cu ion (CuB) to heme a3 (Fea3 and surrounding N atoms) in left figure of Fig. 1(b).
《Glossary》
*1 SACLA
SACLA is a Japanese X-ray free-electron laser (XFEL) facility and was constructed jointly by RIKEN and JASRI. It is a system for generating an X-ray laser from electrons oscillating coherently. SACLA has been developed as one of the national core technologies. Its construction and preparation was launched in FY2006 in a five-year project and was completed in March 2011. The name SACLA is short for SPring-8 angstrom compact free-electron laser.
*2 Radiation damage
Artificial alterations of materials depending on X-ray irradiation are collectively called radiation damage. In general, there are primary radiation damage as a result of the direct interaction of X-rays and the material and secondary radiation damage. The secondary damage is caused by the reactive molecules generated by the primary damage. The most common radiation damage in protein crystallography is caused by chemical reaction between proteins and reactive molecules generated from water on a picosecond timescale upon X-ray irradiation.
*3 High-accuracy high-speed time-resolved structural analysis method
A method of structural analysis by focusing on every minute of functioning proteins. Because the emission time of XFEL pulses produced at SACLA is 10 fs, structures with a lifetime longer than 10 fs can, theoretically, be analyzed. To realize such analysis, it is necessary to carry out experiments by combining this method with a technique for synchronizing the actions of all proteins in a crystal and simultaneously activating them.
*4 Synchrotron radiation
Synchrotron radiation is an electromagnetic wave emitted in the traveling direction of relativistic charged particles (electrons and positrons) bent in a magnetic field. It has excellent properties, such as high brightness, high directivity, and variable polarization properties.
Refer to: http://www.spring8.or.jp/en/about_us/whats_sr/
*5 SPring-8
SPring-8 is a RIKEN facility that generates the world's highest-performance synchrotron radiation and is located in Harima Science Garden City in Hyogo prefecture. The name SPring-8 is short for Super Photon ring-8 GeV.
Refer to: http://www.spring8.or.jp/en/about_us/whats_sp8/
*6 Active site
A specific site of proteins at which enzymatic reaction occurs. At an active site, there are catalytically functional groups promoting enzymatic reaction, such as side chains of polar amino acids and electron-rich metal ions. Because these functional groups themselves are reactive and easily attacked by reactive molecules generated from water upon X-ray irradiation, the active site is susceptible to radiation damage.
|
For more information, please contact: |
- Previous Article
- 100-fold increase in X-ray laser power density (Press Release)
- Current article
- Discovery of a New Crystal Structure Family of Oxide-Ion Conductors NdBaInO4 (Press Release)