X-ray diffraction recordings from a single myofibril
- Release Date
- 06 Aug, 2002
- BL45XU (RIKEN Structural Biology I)
August 6, 2002
Japan Synchrotron Radiation Research Institute (JASRI)
|
The research group led by Dr. Hiroyuki Iwamoto, senior scientist at JASRI, succeeded in recording X-ray diffraction patterns from a single myofibril (diameter, only ~ 2 µm) in a muscle cell by using the RIKEN BL45XU beamline at SPring-8, and in visualizing directly its protein lattice structure. The study was carried out in collaboration with Dr. Tetsuro Fujisawa of RIKEN Harima Institute. |
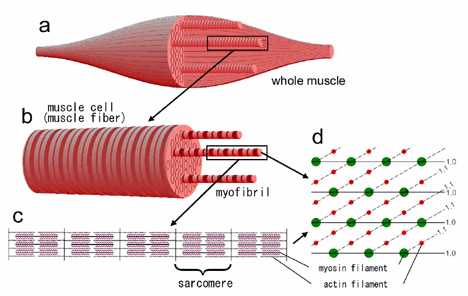
A skeletal muscle (striated muscle) consists of many muscle cells (muscle fibers, diameter, 50 - 100 µm)(Fig. 1a). A muscle cell in turn consists of many myofibrils (diameter, 1 - 2 µm)(Fig. 1b). Each myofibril is made up of many sarcomeres (the smallest functional unit of muscle; length, ~2 µm) connected in series (Fig. 1c). In each sarcomere, the myofilaments (polymer of contractile protein molecules, either actin or myosin) are arranged in a regular hexagonal lattice (Fig. 1d). Because of this arrangement, a number of reflections indexed to the hexagonal lattice can be recorded when irradiated with X-ray beams. The obtained diffraction pattern contains rich information about the structure of the contractile proteins.
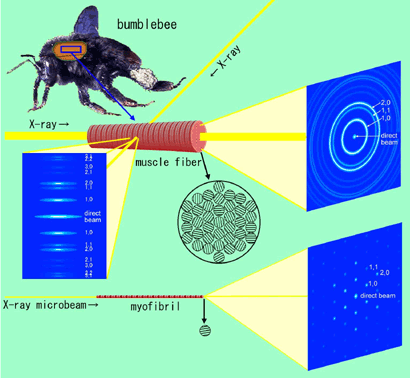
Previously, the smallest muscle specimen from which X-ray diffraction patterns could be recorded had been single muscle fibers. The diffraction pattern recorded from a single muscle fiber (equatorial reflections) are essentially a rotary-averaged pattern from which information about the orientations of the lattice planes has been lost (Fig. 2b, c). This is because a single muscle fiber still contains thousands of myofibrils, and their lattice planes are randomly oriented. To separate the reflections arising from different lattice planes, one must record a diffraction pattern from a single myofibril.
The flight muscle of insects, such as bumblebee (Fig. 2a), is known to have unusually regular arrangement of contractile proteins in the sarcomere. By using this muscle, the research group was able to record diffraction patterns from single myofibrils and to separate reflections arising from different lattice planes of a single lattice (Fig. 3b, c). The patterns were obtained from a specimen whose volume was only 1/1,000 of that of the previously used smallest muscle specimen (single muscle fibers).
The technical background for this achievement is as follows:
(1) The use of X-ray microbeams generated by pinholes (diameter, 2 µm). X-ray microbeams have been used to record diffraction patterns from human hair and other dried biological macromolecules. However, hydrated intracellular protein assemblies are much more susceptible to radiation damage and this is the first example that clear diffraction patterns were recorded by X-ray microbeams from such assemblies. The exposure time was only 5 s. Such a short exposure time was made possible by the high-flux, low-emittance beams from the third-generation synchrotron facility and sensitive detectors. The BL40XU beamline (which became operational recently) can produce even brighter X-ray beams, and its use may make possible the real-time observation of the structural changes of contractile proteins in a lattice.
(2) End-on diffraction. To record diffraction patterns from fibers, the specimen is usually placed perpendicular to the incident X-ray beams. In case of recording from a single myofibril, diffraction theories predict that reflections from only one lattice plane will be recorded at the same time. To circumvent the problem, the myofibril was irradiated end-on along its axis (Fig. 2c, d). This method of diffraction recording has a merit that one does not have to isolate a single myofibril from a muscle fiber, and all that is needed is to shoot at a myofibril in a muscle fiber (although it is also technically difficult). By using this method, one can record reflections from all lattice planes at the same time.
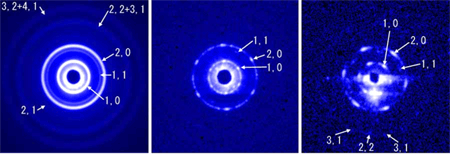
In the diffraction patterns recorded in this way (Fig. 3b, c), the reflections from each lattice plane appear as isolated spots. This is surprising when one considers that the length of the specimen in the beampath is ~3 mm. This is because, in order for the reflections to appear as spots, the lattice planes of the ~1,000 sarcomeres in the 3 mm long myofibril must be strictly in register. Even a 0.1° twist between two neighboring sarcomeres would reduce the diffraction pattern to a rotary-averaged picture as shown in Fig. 2c. The length of 3 mm is almost equal to that of the whole length of the flight muscle of bumblebee. Therefore, one may say that a myofibril in the flight muscle of bumblebee is a single crystal grown in the body of the insect.
Besides the actin-myosin contractile apparatus, there are many other functional protein assemblies in the cell, such as mitotic spindles, cilia, flagella and membrane skeletons. However, they are much smaller than the contractile apparatus of muscle (mostly in micrometer ranges) and their structural analysis by X-ray diffraction has been impractical. However, the present achievement makes it a real possibility that the structure of these protein assemblies can be analyzed by X-ray diffraction. In the post-genome era, it will be increasingly important to analyze the structure and function of proteins in large-scale assemblies. The techniques used here will serve as powerful tools to achieve these goals.
The present study was performed, in collaboration with Dr. Tetsuro Fujisawa at RIKEN Harima Institute, as a part of the research project under SPring-8 Joint Research Promotion Scheme of Japan Science and Technology Corporation.
Explanation of technical terms
Actin and myosin:
The two major contractile proteins in muscle. Each protein polymerizes to form myofilaments. Muscle contraction occurs as the actin and myosin filaments slide past each other. Actin is a globular protein. Myosin consists of a globular head and a filamentous tail region. The head contains binding sites for actin and ATP, which servers as energy source. The tail can polymerize to form the myosin filament backbone.
Hydration:
A state of material in which, as in living cells, its constituent molecules interact strongly with water. In case of enzymes, they are capable of enzymatic reactions while hydrated.
Membrane skeleton:
Cytoskeleton lying immediately beneath the plasma membrane. It serves to keep the shape of the cell. Major constituent proteins include actin and spectrin.
<<Figures:>>
a: a whole muscle. It consists of many muscle cells (muscle fibers).
b: a muscle fiber. Diameter, 50-100 μm. It consists of many myofibrils (diameter, 1-2 μm).
c: A magnified view of a myofibril, made of 2-2.5 μm-long sarcomeres connected in series. In a sarcomere, the myofilaments, made of the contractile proteins actin and myosin, are arranged in a hexagonal lattice (d). A single sarcomere contains a single filament lattice equivalent to a single crystal. The numbers in (d) such as 1,0 and 1,1 represent indices for specific lattice planes.
patterns from a muscle fiber isolated from bumblebee flight muscle
(the diffraction patterns in the figures are also schematic drawings).
a: a bumblebee.
b: Diffraction recording by a conventional method (equatorial reflections. The specimen is placed perpendicular to the incident X-ray beam). Because the myofilament lattices of the myofibrils in a muscle fiber are randomly oriented, reflections originated from all lattice planes are recorded at the same time. However, the information about the orientation of the lattices is lost and the reflections having close lattice constants overlap with each other.
c: End-on diffraction recording from a muscle fiber. X-ray beams are irradiated along the fiber axis. If the beam diameter is large, the diffraction pattern consists of a set of concentric circles (powder diffraction pattern), and again the information about the orientation of the lattices is lost. This is because, in this case too, a large number of myofibrils are in the beam.
d: End-on diffraction recording from a single myofibril using X-ray microbeams. Unlike in this figure, it is not necessary to isolate a myofibril, and all that is needed is to shoot at a myofibril within the muscle fiber. Because only one single hexagonal lattice is in the beam, the reflections originating from each lattice plane appear as separate spots (This occurs only if the lattice planes in all sarcomeres are in register). If the conventional method of recording were applied to a single myofibril (Incident beams are made perpendicular to the fiber axis), it would be impossible to record all the reflections in a single exposure.
(modified from H. Iwamoto et al., Biophys. J., 83:1074-81, Fig. 2)
a: End-on diffraction pattern recorded with a 50 μm-diameter X-ray beam (about the diameter of a muscle fiber). This corresponds to the diagram in Fig. 2c. The reflections appear as concentric circles because of a large number of myofibrils in the beam.
b,c: End-on diffraction pattern recorded with a 2 μm-diameter X-ray microbeam (about the diameter of a myofibril). Reflections originating from a single hexagonal lattice appear as isolated spots. This implies that the lattices of ~1,000 sarcomeres in the ~ 3 mm beampath are perfectly in register. The dark circle at the center of the diffraction pattern is due to the beamstop to protect the detector from the direct beam.
|
For further information, please contact: for research results, Dr. Hiroyuki Iwamoto for SPring-8, Dr. Masahiro Hara |
- Current article
- X-ray diffraction recordings from a single myofibril