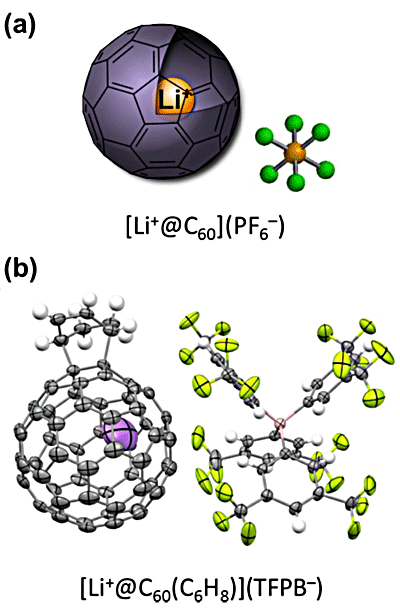Reaction Rate of Fullerene Is Enhanced 2,400-fold by lithium-ion encapsulation (Press Release)
- Release Date
- 17 Jul, 2014
- BL02B1 (Single Crystal Structure Analysis)
The University of Tokyo
Osaka University
Nagoya City University
Key points
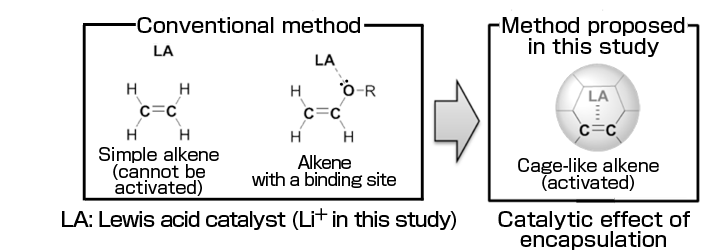
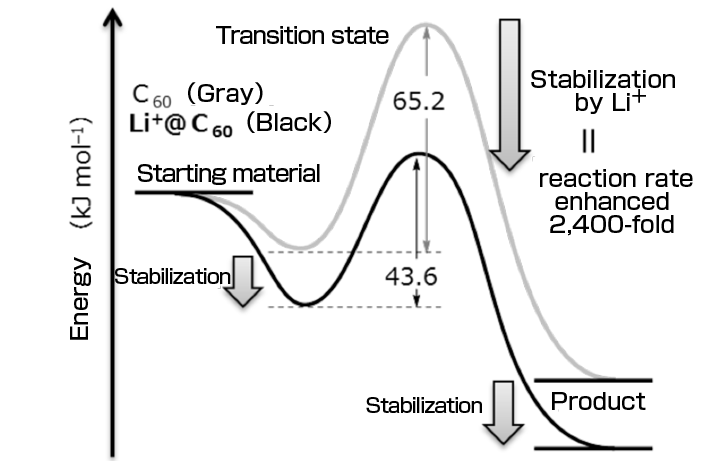
•Quantitatively demonstrating that the Diels–Alder reaction*1 rate of a fullerene, a spherical carbon molecule, was enhanced approximately 2,400-fold by Li+ encapsulation.
•The research group succeeded in determining the sole effect of electronic states on the Diels–Alder reaction for the first time using molecules of exactly the same size with different electronic states, which will lead to the evaluation of optimum conditions for accelerating the Diels–Alder reaction used in the chemical industry and drug synthesis and offer a new concept for catalyst design.
•Obtaining a Li+-encapsulated fullerene derivative as a product, which will be applied to organic solar cells, lithium-ion batteries, and capacitors.
|
Yutaka Matsuo (project professor) of the Graduate School of Science, the University of Tokyo, Ken Kokubo (associate professor) of the Graduate School of Engineering, Osaka University, and Shinobu Aoyagi (associate professor) of the Graduate School of Natural Sciences, Nagoya City University, have quantitatively measured the reaction rate of Li+-encapsulated fullerene(Li+@C60)*2 for the first time in the world. They demonstrated the “catalytic effect of encapsulation” of Li+; namely, the Diels–Alder reaction rate of Li+@C60 was 2,400-fold faster than that of empty fullerene C60*3. Also, while the rate of the Diels–Alder reaction, which is used in the chemical industry and drug synthesis, varies depending on both steric and electronic effects, their method enabled the discussion of the sole electronic effects of the encapsulated Li+ because the empty fullerene and Li+@C60 have the same outer shape and volume, with no difference in steric effects. These results will promote the understanding of the Diels–Alder reaction and provide new guidelines for catalyst design. Also, the Li+@C60 derivative obtained as a product will be used as an organic functional material with a performance exceeding that of other fullerenes and applied to cell materials such as organic solar cells, lithium-ion batteries, and capacitors. Publication: |
《Figures》
《Glossary》
*1 Diels–Alder reaction
An organic synthesis reaction between an alkene with a double bond and a diene with two double bonds resulting in the formation of a six-membered ring product. It was discovered by two German chemists Otto Diels and Kurt Alder, who received the Nobel Prize in chemistry for their discovery in 1950. The Diels–Alder reaction is used in the industrial synthesis of norbornene, an ingredient for polymers, and the synthesis of drugs such as Tamiflu, and also is an important research subject in the area of theoretical chemistry.
*2 Li+-encapsulated fullerene
A novel material encapsulating Li+ in the cage of a fullerene. Currently it is manufactured and distributed by Idea International,a domestic venture company that carries out research and development on fullerene materials.
*3 Fullerene
多A general term for cage-like molecules consisting of a number of carbon atoms. Among them, C60 with the shape of a soccer ball and consisting of 60 carbon atoms is widely used in research as a material for organic solar cells. C60 is industrially manufactured by Frontier Carbon Corporation in Japan.
|
For more information, please contact: Project Prof. Yutaka Matsuo (University of Tokyo) Associate Prof. Ken Kokubo (Osaka University) Associate Prof. Shinobu Aoyagi (Nagoya City University) |
- Previous Article
- Discovery of High-Pressure Minerals in HED Meteorites(Press Release)
- Current article
- Reaction Rate of Fullerene Is Enhanced 2,400-fold by lithium-ion encapsulation (Press Release)