Realization of In Situ and Real-Time Observation of Reaction at Surface of Nanoparticles Using High-Brilliance Synchrotron Radiation at SPring-8 - Success in directly observing the deterioration of electrocatalysts used in fuel cells (Press Release )
- Release Date
- 30 Apr, 2009
- BL16XU (SUNBEAM ID)
- BL28B2 (White Beam X-ray Diffraction)
Japan Synchrotron Radiation Research Institute (JASRI)
NEC Corporation
|
Scientists at the Japan Synchrotron Radiation Research Institute (JASRI; Akira Kira, Director General), working jointly with NEC Corporation (NEC; Kaoru Yano, President), have developed a real-time method of observing changes in the surface structure of nanoparticles in aqueous solution using SPring-8, and have successfully clarified the mechanism behind the deterioration of electrocatalysts used in fuel cells* at the atomic level. The method developed by JASRI and NEC uses energy-dispersive X-ray absorption fine structure (XAFS) spectroscopy (Fig. 1) and high-energy X-ray diffraction, enabling the millisecond-interval observation of the surface structure of catalytic nanoparticles, where the chemical reaction occurs. The scientists investigated the deterioration of Pt catalysts used in fuel cells using this method, and observed an irreversible change from phase α to phase β in the crystal structure of the oxidation layer at the surface of Pt nanoparticles, which involved an expansion of the crystal volume. They clarified that this caused the elution of Pt and led to a deterioration in the performance of fuel cells (Fig. 2). The research and development of fuel cells to improve their performance and reliability is being promoted worldwide because they can generate electrical energy using oxygen from the air and hydrogen from a source and contribute to easing global problems related to the environment and energy. The performance of fuel cells depends on the electrocatalyst, which comprises Pt nanoparticles to promote the electrochemical reaction between hydrogen and oxygen; thus, improving the performance of catalysts and extending their lifetimes are key aims in the development of fuel cells. However, there is a problem in that the Pt components of electrocatalysts elute during the operation of fuel cells, causing the catalysts to deteriorate, which makes the practical application of such fuel cells difficult. Elucidation of the reasons why elution occurs is required to enable practical application, but unfortunately, no methods appropriate for the direct investigation of phenomena occurring on the surface of catalysts in aqueous solutions have been established thus far. The method developed by JASRI and NEC in this research enables the direct observation of the catalytic action of nanoparticles in aqueous solutions at the atomic level. We predict that this method will contribute to the development of fuel cells with high stability and reliability. The achievements of this research were published in the Journal of the American Chemical Society on 6 May 2009. Publication: |
<Glossary>
*Fuel cell
A device that converts the energy produced from the chemical reaction between hydrogen and oxygen into electrical energy. The fuel cells comprise an electrode into which hydrogen is fed and an electrode into which air (containing oxygen) is fed. Hydrogen is decomposed into protons at the hydrogen electrode and oxygen reacts with the protons at the oxygen electrode to generate water. Pt-group catalytic nanoparticles are supported on these electrodes to promote the reaction. The electrodes are insulated using a solid electrolyte film with proton conductivity.
<Figure>
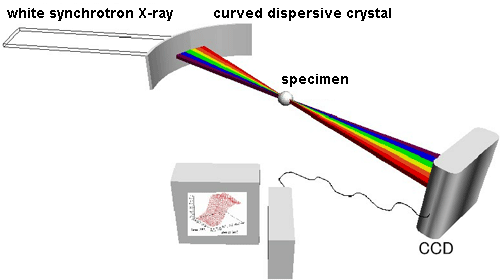 Fig. 1 Principle of energy-dispersive XAFS spectroscopy.
Fig. 1 Principle of energy-dispersive XAFS spectroscopy.Energy-dispersive XAFS spectroscopy is a method of measuring XAFS spectra in a short time using the fact that the diffraction angle depends on the energy of the X-ray when a white X-ray is incident to a curved dispersive crystal. A specimen is placed at the focal point of the X-ray. A position-sensitive detector, such as a CCD, is used as the detector. The measurement can be carried out in a short time of less than 0.001 s because no mechanical operations are required during the measurement.
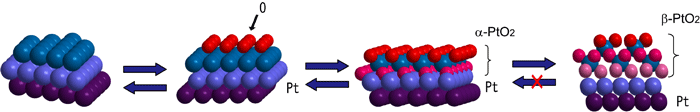 Fig. 2 Structural change due to oxidation of Pt surface.
Fig. 2 Structural change due to oxidation of Pt surface.Phase α of Pt oxide is reversibly reduced to Pt. Phase β is irreversibly reduced to form a disordered structure on the Pt surface. The transition from phase α to phase β is accordingly considered to directly cause the deterioration of the catalysts.
|
For more information, please contact: |
- Previous Article
- Observation of structure of early-stage dental caries at atomic level using high-brilliance X-rays at SPring-8 (Press Release)
- Current article
- Realization of In Situ and Real-Time Observation of Reaction at Surface of Nanoparticles Using High-Brilliance Synchrotron Radiation at SPring-8 - Success in directly observing the deterioration of electrocatalysts used in fuel cells (Press Release )
 .
.