Realizing the World's First Electrolytic Solution Performance in Solid Form at Room Temperature for Use in Batteries (Press Release)
- Release Date
- 18 May, 2009
- BL02B2 (Powder Diffraction)
The creation of nanoparticles was the key to success and will pave the way to developing new safe and high-performance batteries.
Kyushu University
Japan Science and Technology Agency
RIKEN
Japan Synchrotron Radiation Research Institute
|
Scientists at Kyushu University (Setsuo Arikawa, President), Japan Science and Technology Agency (JST; Koichi Kitazawa, President), RIKEN (Ryoji Noyori, President), and the Japan Synchrotron Radiation Research Institute (JASRI; Akira Kira, Director General) have jointly succeeded in developing the world's first solid electrolyte with very high ion conductivity*1 even at room temperature as well as high stability and high robustness to heat in the atmosphere. This new electrolyte is expected to accelerate the realization of unprecedentedly stable and high-performance rechargeable batteries. This result was achieved in joint research by Hiroshi Kitagawa, invited professor, and Rie Makiura, specially appointed research associate, of Kyushu University, and Masaki Takata, chief scientist (also manager of the Research & Utilization Division, JASRI) and Ken-ichi Kato, scientist (also assistant chief scientist at JASRI) of RIKEN. Silver iodide (AgI) has long been known as a highly conductive solid substance (superionic conductor). AgI in the superionic conduction state has high ion conductivity equivalent to that of the electrolyte in lead-acid batteries, i.e., aqueous sulfuric acid solution, typically used in automobile batteries. Unfortunately, this property appears only at high temperatures (at lowest 147°C); this has been a barrier preventing the application of AgI to rechargeable batteries. In this research, the above scientists succeeded in producing special AgI particles that can maintain their superionic conduction state even when the temperature decreases to around room temperature, using nanotechnology. This temperature is the lowest reported so far for AgI-related substances. In addition, the special AgI particles are very stable in the atmosphere and their performance does not deteriorate even after repeated heating. The results of this research are expected to accelerate the realization of safe and high-performance solid-state rechargeable batteries. This research was carried out under the research theme "Creation of the Metal-Organic Hybrid Protonics and Functional Nano-Layer Integrated System" (leader: Hiroshi Kitagawa, professor invited from Kyushu University) in the research area "Development of the Foundation for Nano-Interface Technology" supported by the Core Research for Evolutional Science and Technology (CREST) program of JST. Also, this research study was accepted as a General Proposal to use the large-scale photonic radiation facilities of SPring-8. The original paper describing this research achievement was published in the online version of the British scientific journal Nature Materials on 17 May 2009. Publication: |
<Glossary>
*1 Ionic Conductivity
The property that ionized substances (atoms or molecules in an ionic state) can move in a solid or liquid. Ionic conductivity is expressed as the ionic conduction level (S/cm); solids/liquids with high ionic conductivity are suitable for batteries because their ionized substances can move rapidly.
<Figure>
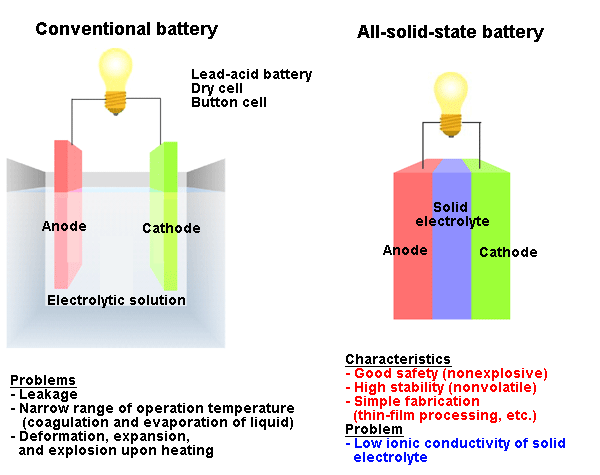 Fig. 1 Schematics of batteries and characteristics of all-solid-state battery.
Fig. 1 Schematics of batteries and characteristics of all-solid-state battery.Most commercially available batteries use a liquid electrolyte because of its high ionic conductivity. However, there is strong demand for the development of all-solid-state batteries from the viewpoints of safety and stability.
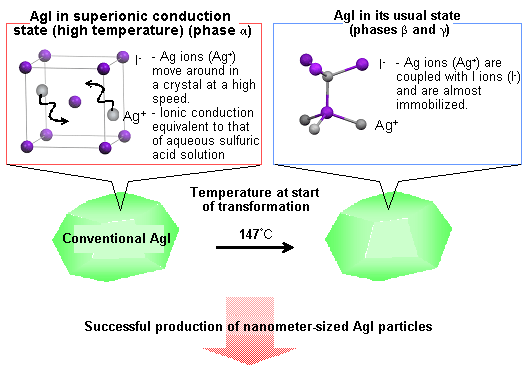 Fig. 2 Characteristics of conventional AgI and AgI nanoparticles developed in this research.
Fig. 2 Characteristics of conventional AgI and AgI nanoparticles developed in this research.(a) Ag ions (Ag+) can move around at high speed in a AgI crystal in the superionic conduction state.
The conventional bulk AgI transforms into a low-ionic-conduction state at 147°C or lower.
(b) The AgI nanoparticles successfully developed in this research were found to maintain their superionic
conduction state even at room temperature. In addition, the developed AgI nanoparticles have ionic
conductivity at least 100,000 times higher than that of conventional AgI at temperatures as low as 4°C.
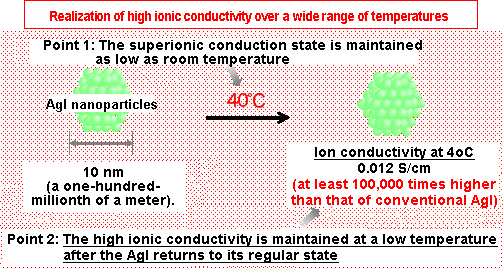 Fig. 3 Schematic of silver-ion solid-state battery.
Fig. 3 Schematic of silver-ion solid-state battery.This battery is composed of solid electrodes and a solid electrolyte, both of which are stable in the atmosphere. Such all-solid-state batteries are considered ideal because there is no liquid leakage, negligible electric leakage or deformation, and good workability in various shapes. Ag ions (Ag+) move rapidly in a solid electrolyte, enabling rapid charge and discharge.
|
For more information, please contact: Dr. Rie Makiura (Kyushu University) Prof. Masaki Takata (RIKEN and JASRI/SPring-8) |
- Current article
- Realizing the World's First Electrolytic Solution Performance in Solid Form at Room Temperature for Use in Batteries (Press Release)
 ,
, ,
, .
.