Development of a New Experimental Method to Clarify Atomic Orbitals of Conduction Electrons in Solids
- Release Date
- 23 Apr, 2010
- BL19LXU (RIKEN SR Physics)
Osaka University
RIKEN
Industrial Technology Center of Wakayama Prefecture
Ritsumeikan University
Japan Synchrotron Radiation Research Institute
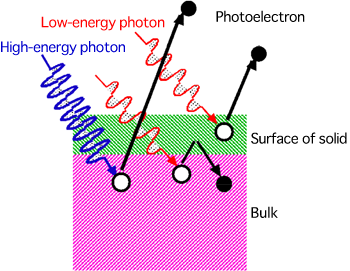
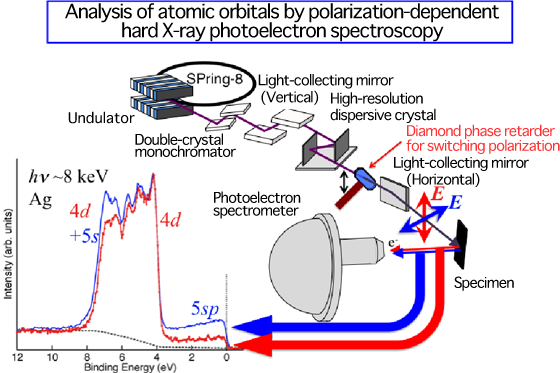
A research group led by Akira Sekiyama, a professor of the Graduate School of Engineering Science, Osaka University (Kiyokazu Washida, President), who is also working as a guest researcher at RIKEN (Ryoji Noyori, President), has succeeded in developing a new method of clarifying the atomic orbitals of conduction electrons in a material by polarization-dependent hard X-ray photoelectron spectroscopy at SPring-8. The group clarified that the properties of conduction electrons are essentially different between gold and silver. This was achieved by joint research with Atsushi Higashiya, a research scientist of the Industrial Technology Center of Wakayama Prefecture (also a guest researcher at RIKEN); Shin Imada, a professor of the College of Science and Engineering, Ritsumeikan University; Tetsuya Ishikawa, the director, Kenji Tamasaku, a senior research scientist, Makina Yabashi, a research scientist (also a research scientist of Japan Synchrotron Radiation Research Institute (JASRI)) of RIKEN SPring-8 Center; and Igor A. Nekrasov, the executive chief scientist of the Institute of Electrophysics, Ural Division of the Russian Academy of Sciences. In this research, the scientists developed a method of directly observing conduction electrons by photoelectron spectrometry with hard X-rays to be irradiated to a material with different polarization directions using the RIKEN synchrotron radiation (SR) Physics Beamline BL19LXU at SPring-8. This enabled the analysis of the atomic orbitals of conduction electrons in a bulk specimen, which was conventionally difficult to verify experimentally. From the measurement of the atomic orbitals of gold and silver, it was clarified that they are greatly different in terms of the atomic orbitals of conduction electrons as well as in color. This is considered to be related to the difference in their resistance to rust. It is expected that the method developed in this research will also contribute to the design of materials in the future. The results of this study were published in the online academic journal, New Journal of Physics, jointly issued by the Institute of Physics (Britain) and Deutsche Physikalische Gesellschaft (German Physical Society) in April 2010. Publication: |
<<Figures>>
photoelectron spectroscopy successfully developed in this research
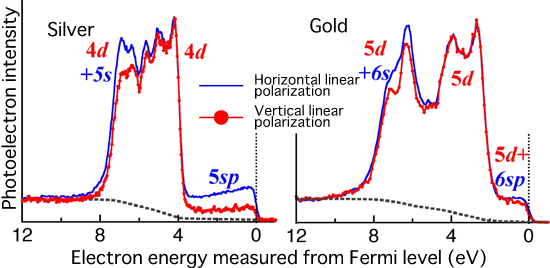
spectra of gold and silver obtained in this research
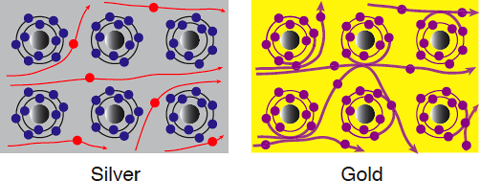
The photoelectron intensity of silver greatly changes at an energy of approximately 0 because of polarization, whereas a negligible change in the photoelectron intensity of gold is observed even when the polarization direction is changed. From this result, the conclusion illustrated in Fig. 4 is obtained.
In the left figure (silver), blue circles represent the d-orbital electrons and red circles represent the s- and p-orbital conduction electrons. In the right figure (gold), the d-, s-, and p-orbital electrons intermingle and are indistinguishable.
|
For more information, please contact: Dr. Kenji Tamasaku (RIKEN) Prof. Shin Imada (Ritsumeikan University) |
- Previous Article
- Development of X-Ray Microscope Enabling Visualization of Electron Density Distribution of Materials with Nanometer Resolution (Press Release)
- Current article
- Development of a New Experimental Method to Clarify Atomic Orbitals of Conduction Electrons in Solids