Success in Controlling Material Properties by Ion Exchange - Providing a guideline for developing new types of memory device (Press Release)
- Release Date
- 16 Aug, 2010
- BL02B2 (Powder Diffraction)
Japan Synchrotron Radiation Research Institute
University of Tsukuba
|
Scientists at the Japan Synchrotron Radiation Research Institute (JASRI; Tetsuhisa Shirakawa, President) and University of Tsukuba (Nobuhiro Yamada, President) have, for the first time in the world, succeeded in observing a reversible symmetry switch of the “host” *1 framework of a cyanide complex *2 by the exchange of “guest” *1 alkali metal cations. The use of such a symmetry switch enables the switching of the color and nonlinear optical *3 properties of a specimen. In conventional research and development, a host substance is considered to merely serve as a container for guest ions and molecules. However, we can make materials express new functions by exploiting the mutual interaction between host and guest substances. By applying a symmetry switch to memory devices, we expect to pave the way to developing new types of memory device, for example, a device that memorizes information depending on the type and number of ions. The cyanide complex used is a functional material with a small environmental impact because it is composed of only materials abundant on Earth, such as nitrogen, carbon, iron, and cobalt. In addition, the cyanide complex is lightweight and soft, and is therefore highly feasible as a next-generation memory material. In the framework of a cyanide complex, small cavities with a width of 5×10-7 mm are regularly arranged. Guest alkali metal cations can move in and out of these cavities. In this research, at least 30 types of compound were synthesized by combining the host framework of the cyanide complex and the guest alkali metal cations, and their structures were systematically analyzed in detail using high-brilliance X-rays at SPring-8, thus leading to the discovery of a symmetry switch. The achievements of this research were realized by Professor Yutaka Moritomo of University of Tsukuba, who is also a visiting scientist at JASRI; Tomoyuki Matsuda, a researcher at University of Tsukuba, and Jungeun Kim, an associate senior scientist at JASRI. The results of this research were published online in Journal of the American Chemical Society. Publication: |
<Figure>
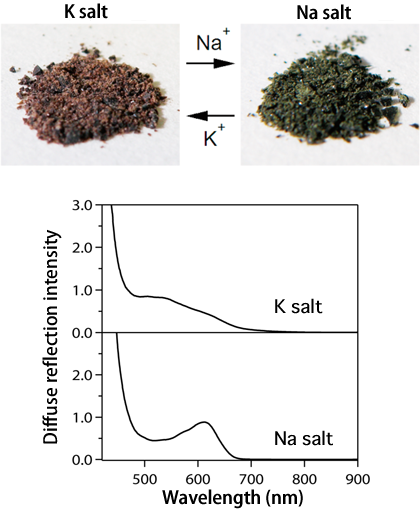
Fig. 1 Images of potassium (K) salt specimen and sodium (Na) salt specimen (upper) and their diffuse reflection spectra (lower).
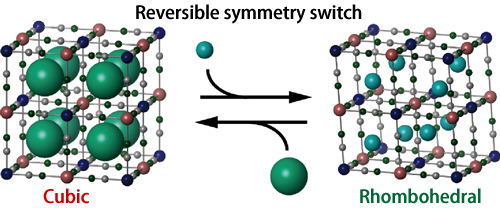
Fig. 2 Host framework of cyanide complex and guest alkali metal cations. The large green spheres represent K ions, and the small spheres represent Na ions. The K salt specimen is cubic, whereas the Na salt specimen is rhombohedral.
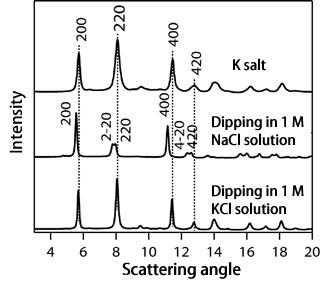
Fig. 3 (Top) X-ray diffraction pattern of K salt specimen. (Middle) X-ray diffraction pattern obtained after dipping K salt specimen in 1 M NaCl solution. (Bottom) X-ray diffraction pattern obtained after subsequently dipping K salt specimen in 1 M KCl solution.
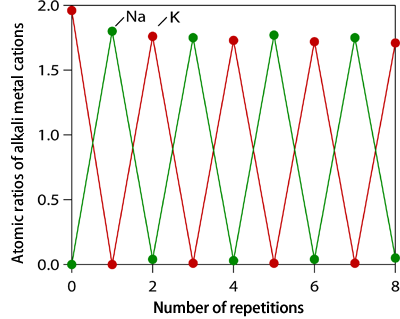
Fig. 4 Atomic ratios of Na and K to Co in specimen alternately dipped in 1 M NaCl and 1 M KCl solutions.
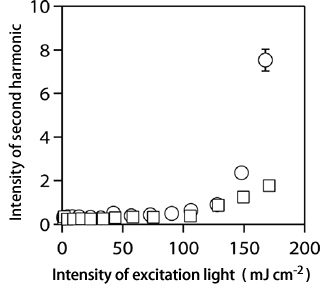
Fig. 5 Dependence of intensity of second harmonic on intensity of excitation light for K salt ( ) and Na salt (
) and Na salt ( ) specimens.
) specimens.
<Glossary>
*1 Host and guest
A substance in which small cavities are regularly arranged is referred to as a host substance, and molecules and ions that can move in and out of such cavities are referred to as guest molecules and ions. For example, in the case of lithium-ion batteries, graphite and cobalt oxide layers are host substances, whereas lithium ions moving in and out of their cavities are guest ions.
*2 Cyanide complex
A cyanide complex is a compound in which transition-metal and iron ions are bridged via cyano groups. When only transition-metal and iron ions are removed from the cyanide complex, a NaCl-type structure (rock salt structure) is obtained. Small cavities with a width of 5×10-7 mm are regularly arranged. A cyanide complex is a functional material with a small environmental impact because it is composed of only materials abundant on Earth, such as nitrogen, carbon, iron, and cobalt.
*3 Nonlinear optics
Nonlinear optics refers to optical responses whose intensity is not proportional to the intensity of light. Second-harmonic generation is a nonlinear optical process. The intensity of the second harmonic is proportional to the square of the intensity of the incident light.
|
For more information, please contact: |
- Current article
- Success in Controlling Material Properties by Ion Exchange - Providing a guideline for developing new types of memory device (Press Release)