Clarification of Structure of Protein Mimicking Transfer RNA - Translation elongation factor P accepts amino acid in reaction similar to that of transfer RNA (Press Release)
- Release Date
- 23 Aug, 2010
- BL41XU (Structural Biology I)
RIKEN
School of Science, The University of Tokyo
Key research achievements
• A protein evolves similarly to transfer RNA (tRNA), a completely different molecule, in terms of shape and reaction.
• The three-dimensional structure of translation elongation factor P (EF-P) that accepts an amino acid from GenX, which evolved from an enzyme that acts on tRNA, is clarified.
• GenX exists only in bacteria and is a promising target for developing new antimicrobial agents without adverse side effects.
|
A joint research team consisting of scientists from RIKEN (Ryoji Noyori, President) and The University of Tokyo (Junichi Hamada, President) succeeded in analyzing the steric structure of a complex composed of translation EF-P, a protein playing an indispensable role in the translation of genetic information, and GenX whose functions remain unclarified. The team also discovered that EF-P accepts an amino acid from GenX. Thus, it was clarified that not only the shape but also the reaction of EF-P is very similar to those of tRNA*1 for the first time in the world. In addition, the transfer of amino acid from GenX to EF-P was found indispensable for the proliferation of Eubacteria*2, such as Escherichia coli. This was achieved by a research team consisting of Shigeyuki Yokoyama, Director of the Systems and Structural Biology Center, RIKEN (also a professor of School of Science, The University of Tokyo); Tatsuo Yanagisawa, a research scientist, and Tomomi Sumida, a special research scientist of RIKEN. To properly synthesize proteins using genetic information of living organisms, it is necessary to select tRNAs that bind to the required amino acids according to the genetic code so that the amino acids are accurately polymerized. This process is called translation; various proteins, for example, aminoacyl-tRNA synthetase (aaRS)*3 and translation factors are involved in this process. The research team has thus far clarified that the shape of translation EF-P involved in the translation initiation is similar to that of tRNA. This time, the team succeeded in crystallizing a complex of EF-P and GenX (a protein whose functions remain unqualified and that is distantly related to aaRS) and clarification of its steric structure; they found that the steric structure is very similar to that of the tRNA-aaRS complex. From this finding, it was clarified that EF-P accepts an amino acid from GenX in a reaction similar to that of tRNA. It was the first discovery of very similar structure and reaction between a nucleic acid and a protein, although they are completely different molecules. This phenomenon seems analogous to convergent evolution, a phenomenon in which different living organisms acquire similar shapes and living behavior through evolution. Furthermore, the transfer of an amino acid from GenX to EF-P was found indispensable for the proliferation of E. coli. GenX exists only in Eubacteria species such as E. coli and Salmonella, and not in eukaryotic organisms*2 such as humans. Therefore, GenX is a promising target for developing new antimicrobial agents for pathogens and antimicrobinal-agent-resistant fungi without adverse side effects. This research was conducted as part of Targeted Proteins Research Program and the results were published on 22 August 2010 in the online version of the American scientific journal Nature Structural & Molecular Biology. Publication: |
<Figure>
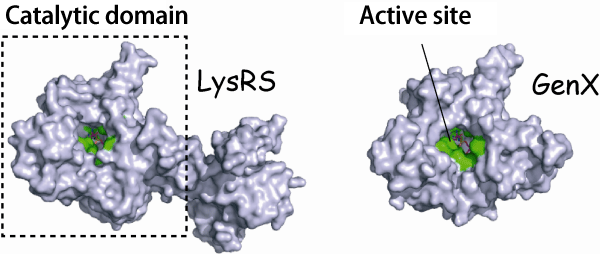
Fig. 1 Structures of LysRS and GenX
The amino acid residues in LysRS and GenX are indicated in green. The structure of the catalytic domain of LysRS is similar to that of GenX.
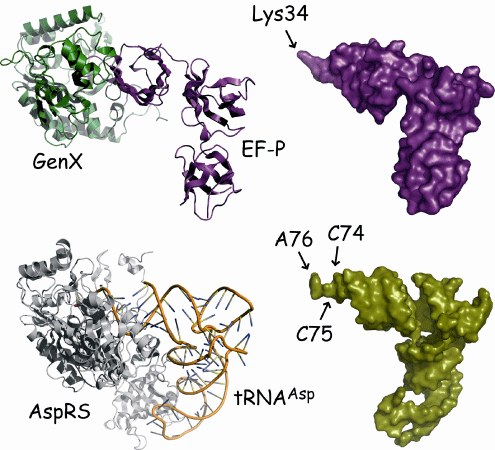
Fig. 2 Structural comparison between EF-P-GenX complex and tRNA-aaRS complex
A complex of asparagine acid tRNA and aspartyl-tRNA synthetase (tRNAAsp-AspRS) is shown as an example. The structures of the EF-P-GenX complex (upper left) and tRNA-aaRS complex (lower left) are very similar. The Lys34 of EF-P corresponds to the CCA terminus (A76) where an amino acid binds to tRNA.
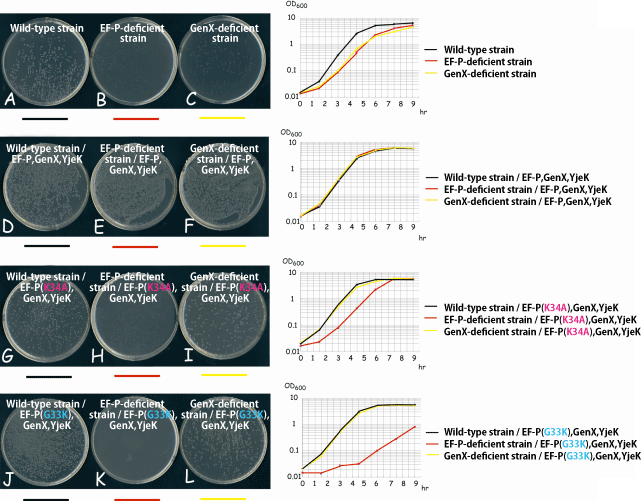
Fig. 3 Effect of lysylation (acceptance of lycine) of EF-P on proliferation of E. coli
Proliferation of E. coli wild-type strain, EF-P-deficient strain, and GenX-deficient strain in agar medium (left) and growth curves in liquid medium (right). The proliferation rates of the EF-P-deficient strain (B) and GenX-deficient strain (C), which are obtained by knocking out EF-P and GenX of the E. coli wild-type strain, respectively, are lower than that of the E. coli wild-type strain (A). However, these proliferation rates are recovered when wild-type EF-P, GenX and YjeK (a protein promoting lysylation of EF-P by GenX, discovered in this research) are added exogenously (E,F). Even when mutant-type EF-P (K34A) or EF-P (G33K), which are not capable of accepting lysyl, is added, the proliferation rate of the EF-P-deficient strain is not recovered (H,K). EF-P in a cell functions with the presence of lysyl. It was clarified that the lysylation of EF-P is indispensable for proliferation of E. coli.
<Glossary>
*1 Transfer ribonucleic acid (tRNA)
tRNA is the abbreviation of transfer ribonucleic acid. One amino acid is coded by trinucleotide units called codons of nucleotide bases of mRNA, namely, adenine (A), guanine (G), cytosine (C), and uracil (U), during translation. The adaptor molecule that relates the information of the nucleotide bases of DNA , i.e., A, G, C, and thymine (T) in triplets, with an amino acid is tRNA. tRNA binds the corresponding amino acid to A76 ribose at the CCA terminus and then is transferred to a ribosome. The amino acids transferred according to the codons on mRNA are polymerized on the ribosome to form a protein.
*2 Eubacteria, eukaryotic organisms
All organisms are classified into three groups: Eukaryota, Archaebacteria, and Eubacteria. Eukaryotic organisms have a nucleus in a cell and the nucleus is separated from other parts of the cell by a membrane. In the nucleus, DNA containing genetic information is contained. Eubacteria are bacteria such as E. coli, Salmonella, Vibrio parahaemolyticus, cholera bacteria, and Shigella. In contrast to eukaryotic organisms, eubacteria have a very simple structure without a nucleus, can exist in any environment on earth, and their metabolic system is diverse.
*3 Aminoacyl-tRNA synthetases (aaRSs), aminoacylation
There are 20 main amino acids that constitute proteins. For each amino acid, a corresponding aaRS exists; for example, AspRS for asparagine acid and LysRS for lycine. After the activation of a specific amino acid by adenosine triphosphate (ATP) energy, the activated amino acid is added to the CCA terminus of a specific tRNA (aminoacylation). aaRS + amino acid + ATP + tRNA -> aaRS.(aminoacyl AMP) + tRNA -> aminoacyl tRNA + aaRS
|
For more information, please contact: |
- Previous Article
- Success in Controlling Material Properties by Ion Exchange - Providing a guideline for developing new types of memory device (Press Release)
- Current article
- Clarification of Structure of Protein Mimicking Transfer RNA - Translation elongation factor P accepts amino acid in reaction similar to that of transfer RNA (Press Release)