First-Ever Success in Crystal Structure Clarification of Enzyme That Catalyzes Nitrous Oxide, a Greenhouse Gas, Generation (Press Release)
- Release Date
- 26 Nov, 2010
- BL26B2 (RIKEN Structural Genomics II)
- BL41XU (Structural Biology I)
- Clue to the Evolution of Respiratory Enzyme from Anaerobic Respiration to Oxygen Respiration
RIKEN
Japan Science and Technology Agency
Kyoto University
Kanazawa University
Key research findings
○ Two iron atoms at the active center are found to convert nitric oxide (NO) to nitrous oxide (N2O).
○ The structural change of the respiratory enzyme molecule, a key in the evolution from anaerobic respiration to oxygen respiration, is clarified.
○ Development of new antimicrobial agents is expected by inhibiting the NO scavenging ability.
|
Scientists at RIKEN (Ryoji Noyori, President), Kyoto University (Hiroshi Matsumoto, President), and Kanazawa University (Shin-ichi Nakamura, President) succeeded in the clarification of the crystal structure of the nitric oxide reductase (NOR) catalyst in the reduction of nitric oxide (NO) to generate a greenhouse gas, nitrous oxide (N2O). Also, they clarified how the catalytic active center consisting of two iron atoms generates N2O, a cause of global warming. This achievement was obtained mainly by Yoshitsugu Shiro, a chief scientist of Biometal Science Laboratory of the RIKEN SPring-8 Center (Tetsuya Ishikawa, Director); So Iwata, the research director (also a professor of Graduate School of Medicine, Kyoto University), and Tomoya Hino (also a visiting researcher at Graduate School of Medicine, Kyoto University), a researcher, of the Iwata Human Receptor Crystallography Project, Explanatory Research for Advanced Technology (ERATO), Japan Science and Technology Agency (JST); and Yoshihiro Fukumori, a professor at Kanazawa University. N2O is the strongest greenhouse gas*1 harming the ozone layer and has an approximately 300-fold higher greenhouse effect than CO2. N2O is produced as a result of the respiration of microorganisms existing in soil and seawater, and the amount of its emission has been increasing annually because of the use of nitrogen fertilizer by humans. N2O is attracting attention in discussions of the global environment of the 21st century. The microorganisms producing N2O respire through denitrification,*2 and obtain the energy to live from nitrogen oxide, such as nitrate ions, instead of oxygen. During the denitrification process, the NOR in the microorganisms generates N2O. The research group succeeded in clarifying the crystal structure of a membrane protein, NOR, of a denitrifying bacteria by X-ray crystallography at SPring-8. In the whole structure consisting of 13 helices penetrating a cell membrane, there is an active center consisting of two iron atoms. Detailed analysis of the structure around these iron atoms revealed the mechanism of the generation of N2O gas. A clue for developing strategic measures to suppress N2O emission by microorganisms can also be obtained. In prehistoric times when there was no oxygen on earth, NOR was indispensable for the respiration of microorganisms. It is considered that NOR evolved into an enzyme for oxygen respiration in the process of the generation of oxygen by photosynthesis that began three billion years ago. Therefore, this result provides important information for considering the scheme for N2O generation, as well as a significant clue for discussing the mechanism of molecular evolution, i.e., how living organisms have changed the molecular structure and function of their respiratory enzyme to accommodate the changes in the global environment. These research achievements were published online in the American scientific journal Science on 25 November 2010. (Publication) |
《Glossary》
*1 Greenhouse gas
Greenhouse gas comprises gas molecules that absorb infrared rays emitted from the surface of the earth and hence raises the temperature of the atmosphere. The most well-known greenhouse gas is carbon dioxide (CO2). Assuming the greenhouse effect of CO2 to be 1, that of methane (CH4) is 21 and that of N2O is as high as approximately 300.
*2 Denitrification
Denitrification refers to the conversion of nitrogen oxide (NOx: nitrate ions (NO3-) and nitrite ions (NO2-)) into nitrogen (N2) gas that is released into the atmosphere. The bacteria carrying out denitrification are called denitrifying bacteria.
《Figure》
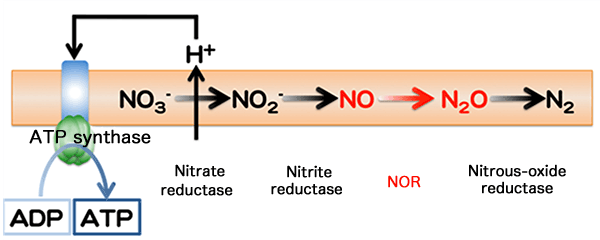
In denitrification, nitrogen oxides (NO3- and NO2-) are sequentially reduced to finally become N2 gas. During this process, NO and N2O are produced. Each step denoted by an arrow → is catalyzed by different enzymes. Membrane-protein nitrate reductase induces a concentration gradient of hydrogen ions through a cell membrane. Using this concentration gradient, ATP synthase catalyzed the synthesis of ATP from adenosine diphosphate (ADP) (nitrate respiration).
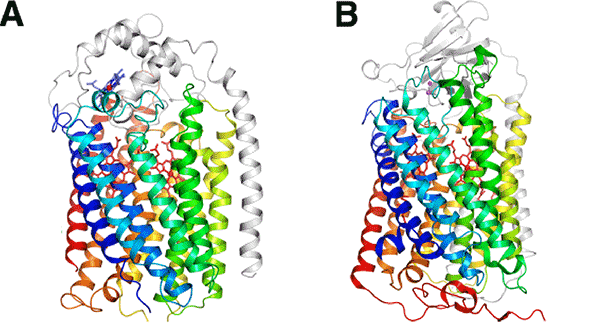
the major subunit of cytochrome oxidase (COX) from bovine cardiac muscle (B)
The arrangement of 13 helices of NOR, penetrating the cell membrane, as indicated by rainbow colors, is very similar to that of a subunit of COX, demonstrating that the ancestor proteins of these two have a common structure.
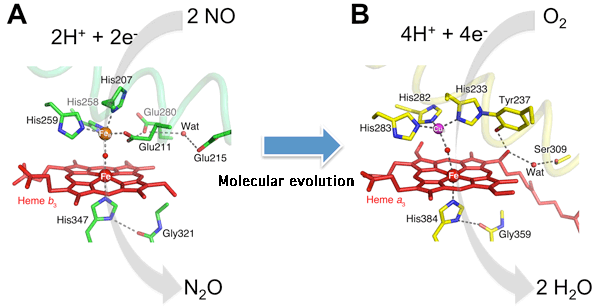
At the active center of NOR, there are two Fe atoms (red and orange balls), where NO is converted into N2O. In COX, one (FeB: orange) of the two Fe atoms is replaced with a Cu atom (CuB: purple). In addition, the type and arrangement of the amino-acid side chains around FeB and CuB are different. Because of this replacement, COX acquired the reaction activity to reduce oxygen.
|
For more information, please contact: |
- Previous Article
- Enabling Collection of Rare Earth Elements by Bacteria - Discovery of Enrichment of Rare Earth Elements by Bacteria (Press Release)
- Current article
- First-Ever Success in Crystal Structure Clarification of Enzyme That Catalyzes Nitrous Oxide, a Greenhouse Gas, Generation (Press Release)