Discovery of Key Site Essential for Regulating the Function of MutL, a DNA Repair Enzyme (Press Release)
- Release Date
- 22 Nov, 2011
- BL45XU (RIKEN Structural Biology I)
RIKEN
Key research achievements
• Discovery of the key site essential for the regulatory mechanism underlying the MutL function in nicking damaged DNA
• Determination of the pathogenesis of Lynch syndrome, a hereditary disorder, by clarifying the mechanism underlying the functional regulation of MutL
• A new finding that may clarify the functional mechanism of the mismatch repair system in DNA damage repair
|
Scientists of RIKEN (President, Ryoji Noyori) have discovered the key site essential for functional regulation of MutL, an enzyme that repairs DNA damage to prevent cells from becoming cancerous. This was achieved by Hitoshi Iino (Postdoctoral Researcher) and Seiki Kuramitsu (Group Director) of the Synchrotron Radiation (SR) System Biology Research Group at RIKEN SPring-8 Center (Director, Tetsuya Ishikawa) and their colleagues. Damage of DNA, the carrier of genetic information in living organisms, causes cancer, aging, and cell death. DNA is constantly damaged by external factors such as ultraviolet light and radiation as well as internal factors such as errors in DNA replication during cell division. Living organisms have several DNA repair mechanisms*1 to cope with various types of DNA damage, among which, the mismatch repair system*2 repairs DNA damage caused by replication errors. Malfunction of this system will lead to the development of Lynch syndrome,*3 which is one of the most frequent hereditary disorders and increases the risk of cancer of multiple organs, such as the colon, endometrium, ovary, and stomach. MutL, an enzyme that plays a key role in the DNA mismatch repair system, is responsible for nicking the damaged DNA; it is considered that the position at which MutL acts and the timing of MutL action are regulated so that MutL does not nick undamaged DNA. Although the mechanism underlying MutL activity has been studied mainly using Escherichia coli and human enzymes, the MutL function in E. coli is different from that in humans, and human enzymes are unstable and not suitable for use in experiments, which remained as problems. The scientists of the research group adopted hyperthermophilic bacterial MutL, which has the same basic functions as those in humans and can be used in experiments because of its stability. They analyzed the structural changes of MutL during the regulation of its function by the hydrogen-deuterium exchange method in combination with mass spectrometry.*4 As a result, the regions related to the functional regulation of MutL were specified, and the key site essential for this regulation was discovered around these regions in an experiment using mutants. They also clarified that the mismatch repair system cannot work when a genetic mutant enters the site, which leads to the development of Lynch syndrome. Further clarification of the functional mechanism of the mismatch repair system will lead to the development of new preventive and treatment methods for Lynch syndrome. Considering that DNA repair is a basic life phenomenon, it is expected that the fundamental DNA repair mechanisms in all living organisms will be more deeply understood. The results of this research were published online in the American scientific journal The Journal of Biological Chemistry on 2 December 2011, prior to publication in the printed version on 9 December. Publication: |
<Figures>
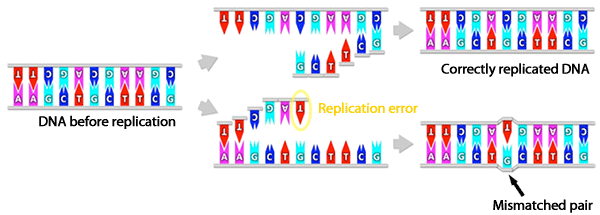
Normally, DNA forms a complementary double-stranded structure, in which adenine (A) binds to thymine (T) and guanine (G) binds to cytosine (C), forming complementary base pairs (A-T and G-C pairs, respectively). However, noncomplementary mismatched base pairs (e.g., G-T) are occasionally formed as a result of errors in DNA replication. The mismatch repair system excises such pairs and repairs DNA.
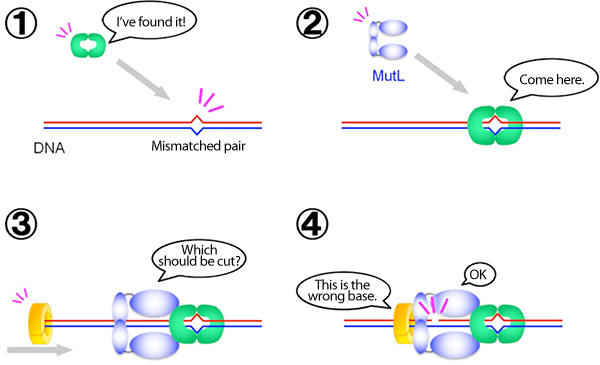
In cooperation with an enzyme that recognizes a mismatched pair of DNA (shown in green) and an enzyme that recognizes the error-containing strand of the DNA duplex (yellow), MutL (blue) nicks only the error-containing strand. This DNA nicking activity of MutL is generally suppressed and is considered to be activated only at a specific time ((4) in the figure).
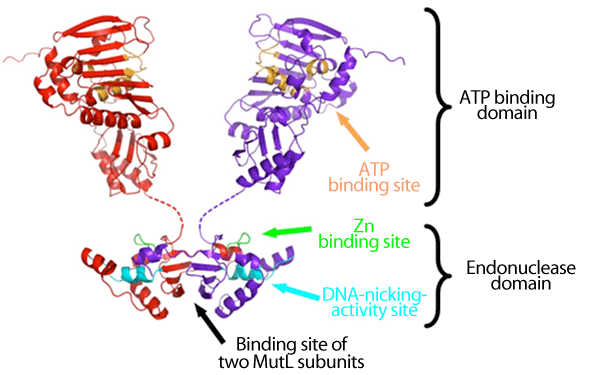
The subunit of MutL has the ATP binding and endonuclease domains, and two MutL subunits (red and purple) are bound via their endonuclease domains. It is considered that when ATP is bound to or unbound from the ATP binding site (orange), the structure of the entire ATP binding domain changes to regulate the DNA-nicking-activity site (cyan) via the Zn binding site (yellow green).
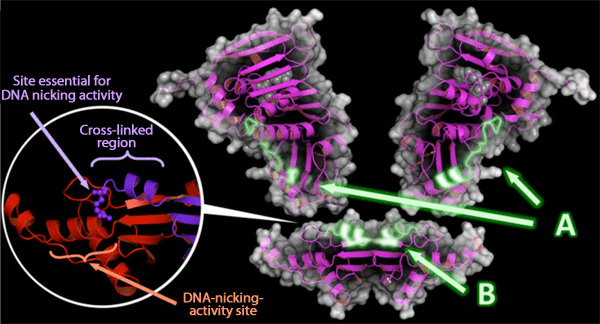
A: Contact region of the ATP binding domain with the endonuclease domain.
B: Contact region of the endonuclease domain with the ATP binding domain.
Circle: Two subunits of MutL (red and purple) are cross-linked and bound within their endonuclease domains. MutL must form a dimer structure to realize a positional relationship that enables the activation of the site essential for DNA nicking activity (purple) and DNA-nicking-activity site (red).
<Glossary>
*1 DNA repair mechanisms
DNA is constantly damaged by external factors such as exposure to ultraviolet light and radiation as well as internal factors such as replication errors and reactive oxygen species. Living organisms have various DNA repair mechanisms that can repair the damaged DNA to its normal state and various DNA repair proteins (enzymes) are involved in these mechanisms. The mismatch repair system is one such DNA repair mechanism.
*2 Mismatch repair system
Mismatch repair is a DNA repair mechanism. DNA forms a complementary double-stranded structure and is therefore capable of self-replication. During DNA replication, the double helix is unwound, and the bases in each DNA strand bind to their new complementary bases, resulting in the formation of two pairs of new double-stranded DNA. However, the replication is not perfect and occasionally generates incorrect double-stranded DNA that contains noncomplementary mismatched base pairs. Such mismatched base pairs are recognized, excised, and repaired by the mismatch repair system.
*3 Lynch syndrome
Lynch syndrome is a hereditary disorder that increases the risk of cancer in multiple organs, such as the colon, endometrium, ovary, and stomach. In particular, Lynch syndrome increases the risk of colon cancer, and has been estimated to be the primary disease in 5-8% of all patients with colon cancer (Lynch and Chapelle (1999) J. Med. Genet. 36, 801-818). In 1966, a case of hereditary nonpolyposis colorectal cancer was reported by Henry Lynch, which was the first reported case of Lynch syndrome. Lynch syndrome is one of the most frequently observed hereditary disorders. Recent advanced research on molecular biology has revealed that Lynch syndrome is caused by the mutations of DNA mismatch repair enzymes.
*4 Hydrogen-deuterium exchange method in combination with mass spectrometry
Deuterium is a stable isotope of hydrogen and has chemical properties similar to those of hydrogen but weighs approximately twofold more than hydrogen. When MutL is immersed in water composed of deuterium (i.e., heavy water), hydrogen in MutL is gradually replaced with deuterium over time. The exchange rate at the surface of MutL is high, whereas those inside MutL and at regions where the domains of its subunits are in contact are low. By fragmenting MutL and measuring the weight of each fragment at the atomic level by mass spectrometry, the structure of MutL can be estimated because the weight of each fragment reflects the hydrogen-deuterium exchange rate.
|
For more information, please contact: |
- Previous Article
- Success in Capturing Instantaneous Atomic Movement Induced by Application of Voltage for Millionths of a Second (Press Release)
- Current article
- Discovery of Key Site Essential for Regulating the Function of MutL, a DNA Repair Enzyme (Press Release)