Dispersion and Orientation Control for Carbon Nanotubes (Press Release)
- Release Date
- 31 Jul, 2012
- BL44B2 (RIKEN Materials Science)
RIKEN
University of Tokyo
University of Tsukuba
Tokyo Institute of Technology
Main points of the results of this research
• Using ionic liquid crystals, we were able to disperse 1000 times as many CNTs compared to using conventional liquid crystals.
• By controlling the orientation of the ionic liquid crystals and CNTs, we successfully controlled the electrical conductivity.
• We are one step closer to the development of soft materials, such as an elastic conductive material, and actuators.
|
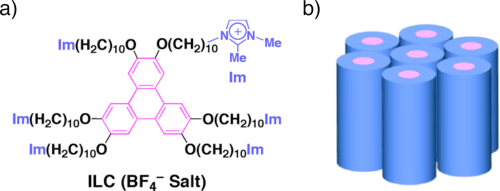
RIKEN (president: Ryoji Noyori), University of Tokyo (president: Junichi Hamada), University of Tsukuba (president: Nobuhiro Yamada), and Tokyo Institute of Technology (president: Kenichi Iga) have succeeded in dispersing 1000 times as many carbon nanotubes (CNTs)*1 as compared to using the conventional method. They also were successful in developing a liquid crystal*2 material whose orientation and electrical conductivity can be controlled. These are the results of a joint research project by Takuzo Aida (professor in the Graduate School of Engineering at the University of Tokyo; also the director of the Functional Soft Matter Research Group of the RIKEN Advanced Science Institute), Jeongho Jay Lee (one of Takuzo Aida’s advanced doctorate students), Yohei Yamamoto (associate professor in the Faculty of Pure and Applied Sciences at the University of Tsukuba), Takanori Fukushima (professor in the Chemical Resources Laboratory at the Tokyo Institute of Technology; also the leader of the Energy Conversion Research Team of the RIKEN Advanced Science Institute), Kenichi Kato (senior research scientist at the RIKEN SPring-8 Center), Masaki Takata (chief scientist at the RIKEN SPring-8 Center), et. al. CNTs, with their superior mechanical and electrical characteristics, have high expectations for use in industrial applications as a new material. However, to take advantage of these characteristics, it is necessary to disperse these CNTs to a high degree, on an individual basis. Using the conventional method, in which CNTs were mixed in with liquid crystals, while it was possible to control the orientation, it was not possible to disperse a sufficient quantity for applications as the CNTs do not have affinity with liquid crystals. Meanwhile, previous research had made it clear that the surface of a CNT is rich in negatively charged pi electrons*3, making CNTs quite affinitive with positively charged ionic liquids*4. This joint research group mixed in CNTs with an ionic liquid crystal containing imidazolium*5, which not only excels in orientability but also has a larger ionized portion than an ionized liquid; the group then discovered that up to around 5 to 10 weight % of CNTs were dispersed extremely efficiently. This is about 1000 times as much compared to the conventional liquid-crystal method. Upon further investigation of the mixture, the group also found out that being mixed with the CNTs, the liquid crystal is vertically oriented; that the orientation of the liquid crystal and CNTs can be independently controlled through shear force*6 and heat; and that the electrical conductivity characteristic can change by up to 2 digits, depending on the orientation of the CNTs. One can hope that in the future this result can be applied in the development of CNT composite materials toward achieving soft electronics*7. The results of this research project were published in the online version of the German science journal Angewandte Chemie International Edition. The paper was selected as a VIP (very important paper) of this journal and was presented on its back cover. Publication: |
<<Figures>>
and (b) a schematic diagram of the structure of a columnar liquid crystal,
in which the molecules are accumulated in columns.
When they are mixed for about 30 minutes at 150°C, a black mixture paste was generated. This mixture maintained its fluidity even when the CNT mixture ratio was raised to 5 to 10 weight-%, and a liquid crystal was formed even at room temperature.
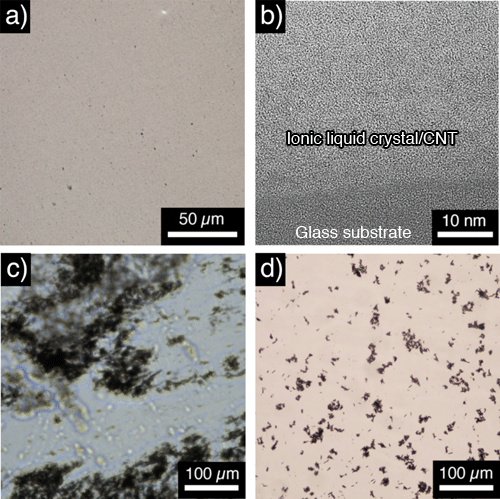
Both (a) and (b) are complexes of CNTs and ionic liquid crystal. Hardly any CNT clusters or bundles are observed; CNTs are highly dispersed in the ionic liquid crystal. (c) is a complex of CNTs with a non-ionic liquid crystal, without positive charge. The liquid crystal and the CNTs are phase-separated, and CNTs are hardly dispersed. (d) shows a complex of CNTs with an ionic liquid; there are some CNT clusters-albeit small-observed.
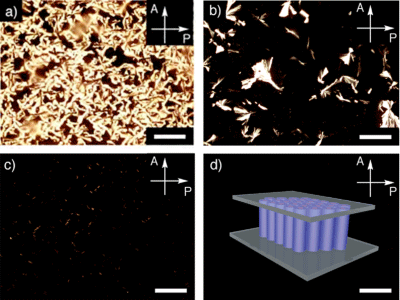
With the addition of CNTs, the liquid crystal columns change from random orientations to vertical orientation. (a) only the ionic liquid crystal; (b) 1 weight-% CNTs were added; and (c) 3 weight-% CNTs were added; (d) 5 weight-% of CNTs were added.
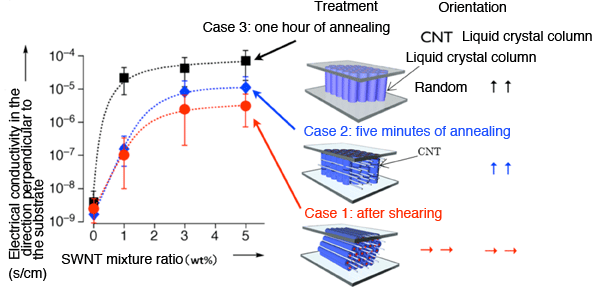
(Left) How the electrical conductivity depends on the CNT mixture ratio, using the orientation-controlled complex of ionic liquid crystal and CNTs at 25°C. The term “SWNT” is an abbreviation for “single-walled carbon nanotubes.” Compared to when the CNTs are randomly oriented (Case 3), the electrical conductivity is about two digits smaller when they are oriented parallel to the plane (Case 1). (Right) The orientations of the CNTs and liquid crystal columns. (Case 1) When a shear force is applied sideways; (Case 2) After 5 minutes of heat treatment; (Case 3) After one hour of heat treatment.
<<Glossary>>
*1 Carbon nanotube
A tube-shaped nano-structure consisting only of carbon atoms. There are single-walled and multi-walled carbon nanotubes. In this research, single-walled carbon nanotubes (SWNT HiPCO) were used.
*2 Liquid crystal
State of matter with properties between a liquid and a solid; it has fluidity and a periodic structure.
*3 Pi electron
An electron in a pi orbital, contributing to a pi bond. It carries electricity in organic conductors and carbon materials.
*4 Ionic liquid
An ionic chemical compound in a liquid state at room temperature.
*5 Imidazolium
A 5-membered ring containing 2 nitrogen atoms (the blue part of Fig. 1a)
*6 Shear force
Force applied to an object in the direction parallel to a plane in its interior, as though sliding along the plane. In this research, a shear force is applied to the test subject by putting it between glass substrates and then sliding the glass while keeping pressure on it.
*7 Soft electronics
Electronics using elastic electric conductor materials. It is characterized by the appearance of new functions that are not possible in hard electronics with conventional, inorganic materials; these new functions include integrating electronic elements on actuators and flexible substrates.
*8 Polarizing microscope
An optical microscope using polarized light as incident light. Liquid crystals have the property of turning the polarization plane, producing double refraction. When a liquid crystal specimen is observed with two polarizers placed so that their polarizing directions are perpendicular to each other (crossed Nicol), one can see a bright field image (Fig. 4a); when the liquid-crystal columns are oriented perpendicularly, double refraction does not occur, creating a dark field image (Fig. 4d).
*9 Anisotropy
When the physical properties of a substance depend on the orientation of the liquid crystal (or change when the liquid crystal is turned), the substance is said to have “anisotropy”; the antonym is “isotropy.”
|
For more information, please contact:
Assoc.Prof. Yohei Yamamoto (University of Tsukuba)
D.Sci. Masaki Takata (RIKEN) |
- Previous Article
- Successful Growth of a Nano-Crystal Thin Film Systematically Oriented with a 3D-Porous Material (Press Release)
- Current article
- Dispersion and Orientation Control for Carbon Nanotubes (Press Release)