Elucidation of Mechanism Underlying Responses of a Receptor to Various Stimuli (Press Release)
- Release Date
- 23 Apr, 2013
- BL26B2 (RIKEN Structural Genomics II)
- BL41XU (Structural Biology I)
RIKEN
Key points
• The structure of the function-regulatory region of TRP channels is elucidated.
• The function-regulatory region flexibly respond to various stimuli through assembly and disassembly.
• The achievement will contribute to understanding of molecular functions of TRP channels, a potential key target of drug development.
|
A group from RIKEN (president, Ryoji Noyori) elucidated part of the mechanism underlying the responses of TRP channels*1, which are receptors present in biomembranes. Each type of TRP channel can flexibly respond to various multiple stimuli. This was achieved by a joint research group of Atsuko Yamashita (team leader, currently a professor at the Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University) and Makoto Ihara (research scientist, currently a lecturer at the Faculty of Agriculture, Kinki University) of the Molecular Signaling Research Team of RIKEN SPring-8 Center (director, Tetsuya Ishikawa); Nobuyuki Uozumi (professor) and Shin Hamamoto (assistant professor) of the School of Engineering, Tohoku University; Masatsune Kainosho (professor), Yohei Miyanoiri (specially appointed assistant professor), and Mitsuhiro Takeda (specially appointed assistant professor) of the Structural Biology Research Center, Nagoya University; and Isamu Yabe (research scientist) of Tokyo Denki University. There are ion channel receptors*2 of transmembrane proteins on cells and organelle membranes (biomembranes) in cells. The receptors receive extracellular stimuli and convert them into biological information. Upon stimulation, the receptors allow ions such as Ca2+ to be transmitted, resulting intracellular signaling. In particular, TRP channels respond to various stimuli. For example, the capsaicin receptor TRPV1, a type of TRP channel, responds to the pungent component in chili peppers (capsaicin) and also responds to temperatures exceeding 42 °C. That is why one feels a burning sensation and pain upon eating chili peppers. Humans have 27 types of TRP channel. It is expected that these TRP channels have regions for transmission of ions of similar structures and functions as well as intercellular regions of various shapes and functions depending on the type. However, the reason why even a single type of receptor can respond to various multiple stimuli has not been clarified in detail. The research group identified a fungal TRP channel, TRPGz, which is assumed as a prototypic model of TRP channels, and used it for analysis. The function-regulatory region of TRPGz was analyzed by X-ray crystallography at SPring-8*3 managed by RIKEN, and nuclear magnetic resonance (NMR)*4 spectroscopy. It was revealed that four modules are assembled into one structure to respond to changes in extracellular osmotic pressure, and that the modules are easily disassembled to respond to other stimuli. This achievement provides significant information for deepening our understanding of various vital responses related to TRP channels and their molecular functions as a target of development of drugs such as analgesics. This research was supported by the Funding Program for Next-Generation World-Leading Researchers by the Japan Society for the Promotion of Science and the Targeted Proteins Research Program by the Ministry of Education, Culture, Sports, Science and Technology. The achievement was published online in the American scientific journal Journal of Biological Chemistry prior to the printed version published on 24 May 2013. Publication: |
<<Figures>>
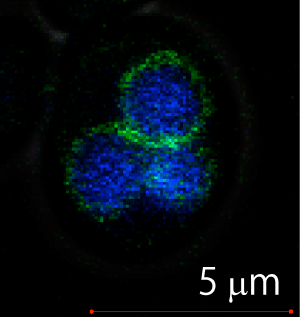
TRPGz (green) is localized on the yeast vacuole (blue) membrane.
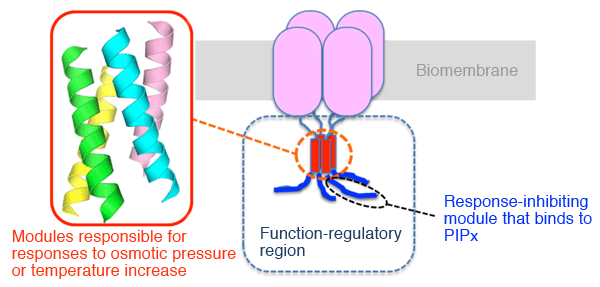
TRPGz (green) exists in the biomembrane.
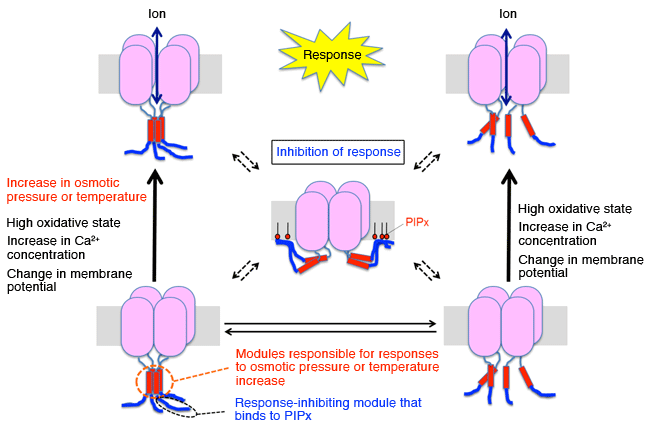
When the modules responsible for response to osmotic pressure or temperature increase assemble, the channel opens (responds) and ions can be transmitted upon the increase in osmotic pressure or temperature (left). When the modules disassemble and other modules called response-inhibiting modules bind to phosphatidylinositol phosphates (PIPx) in the biomembrane, the channel remains closed, thereby inhibiting the response (middle). When Ca2+ concentration or membrane potential increases or in the high-oxidative state, the channel opens (responds) even if the modules responsible for response to osmotic pressure or temperature increase do not assemble (right).
<<Glossary>>
*1 TRP channels
TRP channels are receptors of transmembrane proteins that are related to the perception of various sensations including temperature, pain, and taste sensations. TRP channels serve as a sensor of external stimuli. TRP channels are known to play an important role in receiving various physical and chemical stimuli including visual, taste, olfactory, hearing, tactile, and temperature sensations in humans, rodents, birds, Drosophila, nematodes, and zebra fish.
*2 Ion channel receptors
Ion channel receptors are transmembrane proteins. They have pores through which ions can be transmitted. In addition, they have gates; by their opening and closing, the flow of ions is regulated to induce changes in membrane potential and ion concentration across the membrane for rapid signaling.
*3 SPring-8
SPring-8 is a synchrotron radiation facility that provides the world’s highest-brilliance synchrotron radiation. It is owned by RIKEN and located in Harima Science Park City, Hyogo Prefecture, Japan. JASRI is responsible for the operation, management, and promotion of the use of SPring-8. The name “SPring-8” is derived from “Super Photon ring-8 GeV”. When the direction of the electron beams accelerated to nearly the speed of light is changed by magnets, electromagnetic waves are emitted in the tangential direction; these waves are synchrotron radiation. When the electron beam has a higher energy and the change in the traveling direction is large, synchrotron radiation contains shorter-wavelength lights such as X-rays. In particular, the following three facilities are known as the third-generation large synchrotron facilities: SPring-8 in Japan, the Advanced Photon Source (APS) in the USA, and the European Synchrotron Radiation Facility (ESRF) in France. Because the ring at SPring-8 enables an electron acceleration energy of 8 giga-electronvolts to be generated, synchrotron radiation in a wide range of wavelengths can be obtained including far-infrared light, visible light, vacuum ultraviolet light, soft X-rays, and hard X-rays. SPring-8 is used by researchers both in Japan and overseas for joint research in various fields such as materials science, earth science, life science, environmental science, and industrial applications.
*4 NMR
When a nucleus is placed under a strong magnetic field, magnetic spins of the nucleus align along the magnetic field. When the aligned magnetic spins are irradiated with a specific electromagnetic wave, the electromagnetic wave is absorbed by the spins because of resonance, which is called NMR. The structure of a protein is determined by NMR because the state of electrons around the nucleus and the bonding state of atoms are determined. Unlike crystallography, NMR can determine the structure of a protein even in solutions.
|
For more information, please contact: |
- Previous Article
- Quantitating the surface-strain dominated oxygen-reduction activity of monolayer Pt shell catalysts (Press Release)
- Current article
- Elucidation of Mechanism Underlying Responses of a Receptor to Various Stimuli (Press Release)