Successful Synthesis of Artificial Rhodium (Press Release)
- Release Date
- 22 Jan, 2014
- BL02B2 (Powder Diffraction)
Kyoto University
Japan Science and Technology Agency (JST)
|
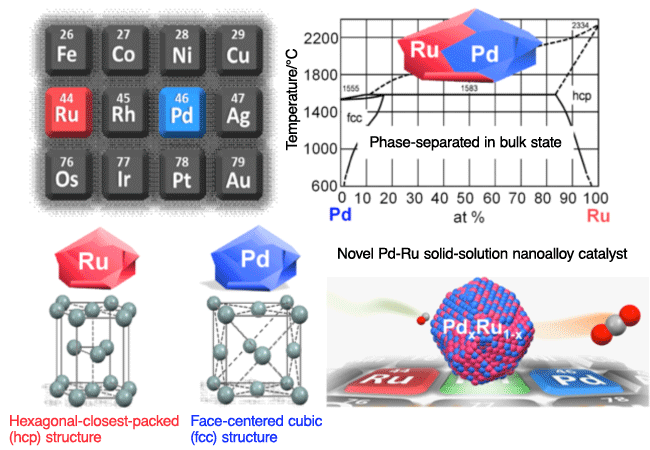
A research group led by Hiroshi Kitagawa (professor) of the Graduate School of Science, Kyoto University, succeeded in synthesizing a new alloy composed of palladium (Pd) and ruthenium (Ru) mixed at the atomic level. Usually Pd and Ru are immiscible at the atomic level because, like oil and water, phase separation*1 occurs between the two elements in the solid and liquid states, even at temperatures in excess of 2,000 °C. By utilizing a unique chemical reduction method, the Kitagawa group has found that the two metals can be mixed into an alloy due to a nano-size effect, forming a solid-solution of Pd and Ru within nanoparticles. Because these alloy nanoparticles have an electronic state equivalent to that of the most expensive metal, rhodium (Rh), which is located between Ru and Pd in the periodic table, it is expected to be used as artificial Rh, but at one-third the cost. Currently, in the Ene-Farm fuel cell cogeneration system for household use, Ru is used as a catalyst to prevent the contamination of the rare metal platinum. This novel alloy catalyst in which Pd and Ru are mixed at the atomic level showed a higher performance for removing the toxic carbon monoxide (CO) compared with the currently used Ru catalyst. This alloy also showed a catalytic activity higher than that of Rh, which is positioned between Ru and Pd, as shown in Fig. 1. In summary, the Kitagawa group succeeded in mixing Pd and Ru and obtained a material that acts like a new element, Pd1- xRux (0<x<1), which had never existed on earth. The durability of the expensive platinum catalyst in fuel cells will be improved with the use of this alloy, resulting in a dramatic improvement in the service life of Ene-Farm. Also, this novel alloy is expected to exhibit a higher catalytic performance than the Rh catalysts that are used for purifying automobile emissions. The cost of the novel alloy is one-third or less than that of Rh, currently the most expensive precious metal. Even when Rh cannot be used because of cost issues, the novel alloy will provide a performance equal to or higher than that of Rh. This research was conducted as part of the project entitled “Creation of Functional Materials on the Basis of the Inter-Element-Fusion Strategy (Research Director, Professor Hiroshi Kitagawa from Kyoto University)” in the research area “Creation of Innovative Functions of Intelligent Materials on the Basis of Element Strategy”, which is supported by Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST). Their achievements will be published online as a rapid communication in Journal of the American Chemical Society, a leading journal in this area, in the coming days. Publication: |
<<Figures>>
are mixed with each other at the atomic level, and its CO-oxidizing activity
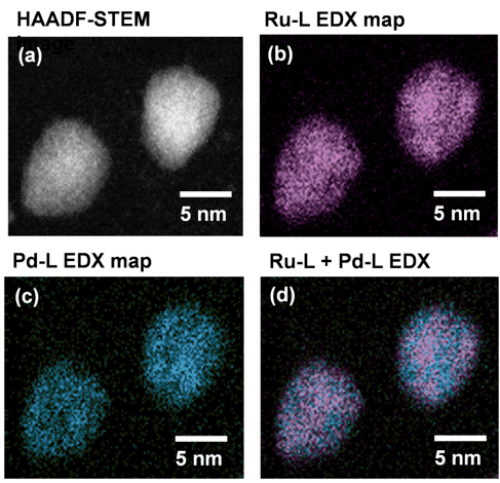
(a) HAADF-STEM image; (b) element mapping of Ru; (c) element mapping of Pd; and (d) superimposition of element maps of Pd and Ru. The superimposition of element maps of Pd and Ru indicates that these are alloy nanoparticles in which Pd and Ru are in solid solution at the atomic level.
solid-solution nanoalloy catalyst and (c) their Rietveld analysis results
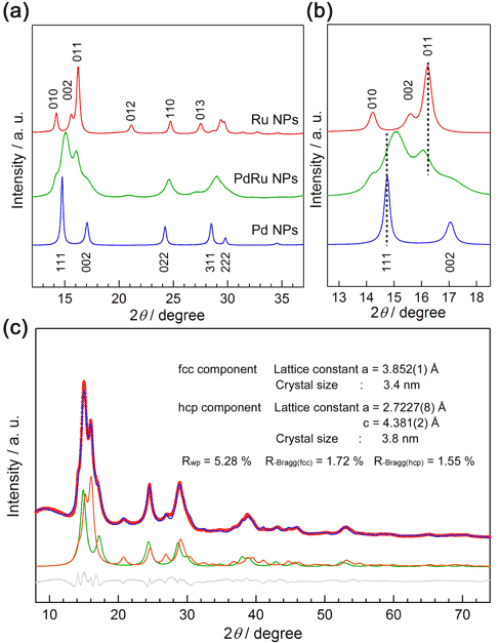
The results of the Rietveld analysis of the powder X-ray diffraction patterns show that the face-centered cubic (fcc) and hexagonal-closest-packed (hcp) structures of Pd-Ru solid solution coexist in the alloy.
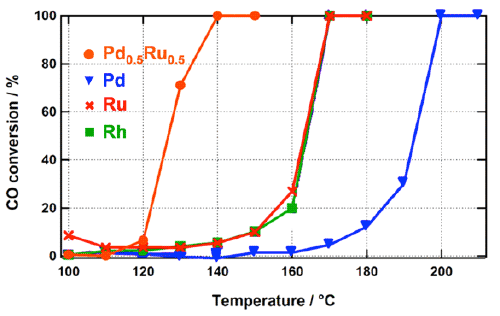
T50 indicates the temperature at which the conversion rate*3 of CO reaches 50%. The novel Pd-Ru solid-solution nanoalloy catalyst shows a higher catalytic activity than Pd, Ru, and Rh particles under mild conditions.
<<Glossary>>
*1 Phase separation
A phenomenon in which substances are separated into two phases. In this research, phase separation indicates a state in which two or more types of metals exist separately without being mixed with each other at the atomic level.
*2 High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM)
A type of electron microscopy in which a finely focused electron beam is scanned over a sample and the transmission electrons scattered at high angles are detected using an annular detector.
*3 Conversion rate
Percentage of reactants that are consumed in the reaction.
|
For more information, please contact: |
- Previous Article
- Low Core-Mantle Boundary Temperature Inferred from the Solidus of Pyrolite (Press Release)
- Current article
- Successful Synthesis of Artificial Rhodium (Press Release)