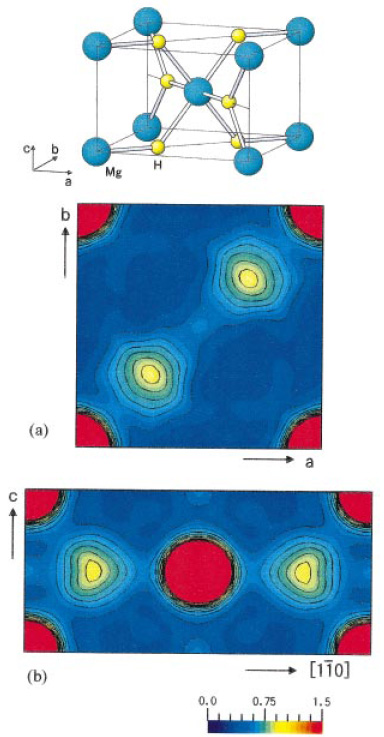
Chemical bonding of hydrogen in MgH2
利用事例本文
Powder diffraction is a powerful technique to study crystal structure. Using this technique, one can measure structural parameters such as lattice parameters, atomic positions, etc of crystalline materials. By using synchrotron radiation one can also obtain charge density level structures closely related with physical properties as well as structural parameters. The figure shows charge density distributions obtained by analyzing diffraction data of a base materials for hydrogen storage, MgH2. These data reveal the fact that hydrogen is weakly bonded to Mg, which must be a big advantage of hydrogenation dehydrogenation of this substance.
Fig. Charge densities of MgH2
[ T. Noritake, M. Aoki, S. Towata, Y. Seno, Y. Hirose, E. Nishibori, M. Takata and M. Sakata, Applied Physics Letters 81, 2008-2010 (2002), Fig. 2,
©2002 American Institute of Physics ]
学術利用キーワード
| A. 試料 | 無機材料 |
|---|---|
| B. 試料詳細 | 金属・合金, 結晶性固体, 結晶 |
| C. 手法 | X線回折 |
| D. 手法の詳細 | 粉末結晶構造解析 |
| E. 付加的測定条件 | 室温 |
| F. エネルギー領域 | X線(4~40 keV) |
| G. 目的・欲しい情報 | 結合状態, 構造解析, 結晶構造, 機能構造相関, 電荷密度 |
産業利用キーワード
| 階層1 | 電池 |
|---|---|
| 階層2 | 燃料電池 |
| 階層3 | 電極 |
| 階層4 | 結晶構造 |
| 階層5 | 回折 |
問い合わせ番号
SOL-0000000951