Clarification of Oxygen Adsorption Mechanism of Oxygen Storage Protein, Myoglobin (Press Release )
- Release Date
- 08 Apr, 2009
- BL17SU (RIKEN Coherent Soft X-ray Spectroscopy)
RIKEN
The University of Tokyo
|
Small amounts of metal elements absorbed in the human body endow proteins with their characteristic functionalities, which are abundant in nature, and such proteins have long been known as metalloproteins. Heme proteins that store and transport oxygen in blood, such as myoglobin and hemoglobin, are particularly well known, and their oxygen storing and releasing mechanisms depending on the oxygen concentration have been studied by various techniques. The mechanisms have been explained as follows. An Fe atom is surrounded by four N atoms on the same plane at the site where oxygen is actually stored - the center of the reaction, called heme - and the Fe atom is thereby called heme-Fe (Fig. 1). During bonding with oxygen, this heme-Fe moves with the fifth N atom outside the plane, by which the heme-Fe enables the reversible storage and release of oxygen. Actually, however, the spin state of heme-Fe changes before and after the adsorption of oxygen; the above reversible phenomenon at room temperature has remained a mystery. To unveil this mystery, scientists focused on the 3d electronic state of heme-Fe, which is directly related to the bond with oxygen in heme proteins. Unfortunately, it had been impossible to experimentally observe the electronic state of an Fe atom embedded in a gigantic protein molecule. Recently, the research group led by Yoshihisa Harada and Shik Shin at the University of Tokyo and RIKEN have developed a new system with which an experimental method, called soft X-ray resonance emission spectroscopy, was adapted to the study of aqueous proteins. They were the first to observe the 3d electronic state of Fe in myoglobin, which contains only one Fe atom in its large molecule with a molecular weight of approximately 16500, using the high-brilliance soft X-rays produced at SPring-8. They clarified that the mechanisms behind the storage and release of oxygen by myoglobin depend on the electronic state of heme-Fe that can easily change its valence and spin state. The achievements of this research were published in the April 2009 issue of the international journal of JPSJ, Journal of Physical Society of Japan. Publication: |
In the soft X-ray resonance emission spectroscopy used in this research, as shown in Fig. 2, X-rays with the energy hνin are first irradiated onto heme-Fe to excite its electron from the inner shell with low energy to the 3d state. An X-ray is then emitted when the vacant inner shell is occupied by the other electron in the 3d state, and its energy hνout is measured. The energy level of the 3d state of heme-Fe is determined by the difference between hνin and hνout. It is possible to excite only the electron of the target Fe by irradiation of X-rays with the appropriate hνin (element selectivity), which is the marked advantage of this method. The scientists compared electronic states among the heme proteins with different adsorbed molecules (X in Fig. 1) as observed by this method. They discovered a fact that overturned the conventional theories, that is, the 3d electron energy level of heme-Fe changes negligibly for all of the heme proteins even though the valence and spin state of heme-Fe differ for each type of molecule. The reasons are considered to be that (1) heme-Fe is placed at the site where the spin transition is easily induced and (2) the five N atoms surrounding Fe, instead of Fe itself, are in charge of changing the valence because of the expansion of the 3d electronic state of heme-Fe. These characteristics of heme-Fe in myoglobin indicate the extremely small change in the electronic state of heme-Fe associated with the adsorption of oxygen and are the basis of the electronic mechanisms that enable the reversible adsorption and desorption. Moreover, these characteristics are similarly observed in the 3d electronic state of transition metal impurities embedded in semiconductors, suggesting the possibility of applying research methods that have long been used in the field of solid-state physics to even proteins in order to clarify the properties of metal centers in human bodies.
This research has attracted the attention of many scientists because it established the experimental method for clarifying the electronic state of metals in aqueous proteins. In the future, we hope that further analysis of metal centers for various proteins will add a new aspect of electronic state analysis to the project on the structural analysis of proteins – called postgenome – and deepen the understanding of the functionalities of proteins.
<Figure>
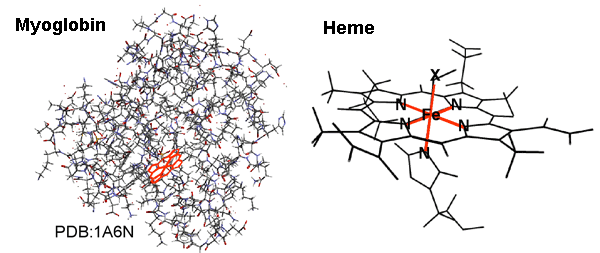 Fig. 1 Example of molecular structure of myoglobin and magnified view of its inner heme.
Fig. 1 Example of molecular structure of myoglobin and magnified view of its inner heme.The heme expresses the function when a small molecule X is reversibly adsorbed to and desorbed from an Fe atom.
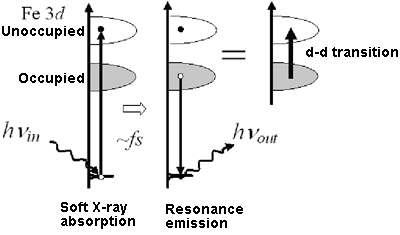 Fig. 2 The 3d electronic state of the Fe atom embedded in a protein can be detected by resonance light scattering with soft X-rays.
Fig. 2 The 3d electronic state of the Fe atom embedded in a protein can be detected by resonance light scattering with soft X-rays.
|
For more information, please contact: |
- Current article
- Clarification of Oxygen Adsorption Mechanism of Oxygen Storage Protein, Myoglobin (Press Release )
 .
.