World's First Clarification of Three-Dimensional Structure of Human Gap Junction Channels - Hopes for contributing to establishment of strategies for medical treatment of deafness and irregular pulse (Press Release)
- Release Date
- 02 Apr, 2009
- BL44XU (Macromolecular Assemblies)
|
Scientists under Tomitake Tsukihara, a specially appointed professor at the Picobiology Laboratory, Graduate School of Life Science, University of Hyogo, succeeded in clarifying the three-dimensional structure of human gap junction channels,*1 for the first time in the world, using the Molecular Assemblies Beamline BL44XU at SPring-8, which is the contract beamline of the Institute for Protein Research, Osaka University. The achievements of this research were published in the British journal Nature on 2 April 2009. This research was supported by a Grant-in-Aid for Scientific Research. Publication: |
Background and achievements of this research
A number of cells aggregate into organs of higher organisms. Such cells work in close association with adjacent cells in each organ. For example, a heart does not start beating unless its myocardial cells work in a coordinated manner. Also, organs, including the inner ear, normally work when their constituent cells, homogeneous or heterogeneous, coordinate their activities with each other. Such intercellular association and coordination are closely related to a special membrane region called gap junction, where many gap junction channels aggregate.
The detailed structure of gap junction channels has not yet been clarified, although several decades have already passed since their discovery. Professor Tsukihara (Picobiology Laboratory, Graduate School of Life Science, University of Hyogo) and his colleagues determined the structure of gap junction channels by X-ray crystal structure analysis*2 using the Molecular Assemblies Beamline BL44XU at SPring-8.
The gap junction channel was found to penetrate two adjacent cellular membranes,*3 and its entire structure resembles a Japanese drum (tsutsumi) (Fig. 1). There is a long cavity with a minimum diameter of 1.4 nm in the center along the long axis of the molecules. Small molecules and ions can pass through this cavity. We hope that the successful identification of the amino acid residues facing the cavity and their positions will markedly accelerate research on the permeability of channels (Fig. 2).
In this study, the structure of an open channel was determined. In 2007, researchers at Kyoto University reported the structure of a closed channel determined by low-resolution electron crystal structure analysis.*4 A large structure was observed at the upper portion of a pore of the closed channel, and it seems to physically block the channel. However, for the open channel revealed in this study, a funnel-shaped structure consisting of six short α–helices was observed at the upper portion of the channel pore, and no obstacle existed on the permeation pathway of the channel (Fig. 3). By examining the above two structures together, we hypothesized that this funnel-shaped structure is closely related to the opening and closing mechanisms of gap junction channels.
Thus far, at least 20 types of connexin, which is the protein that forms gap junction channels, have been identified in human bodies. They are all considered to have a similar structure. It is also well known that most of their mutants are the causes of disorders such as deafness and an irregular pulse. From the atomic structure determined in this study, the researchers clarified the effects of such mutants on the structure and functions of channels. They also elucidated the relationship between the genetic mutation of gap junction channels and the above disorders on the basis of the three-dimensional structure. We hope that these achievements will be used in establishing strategies for treating diseases in the future.
<Figures>
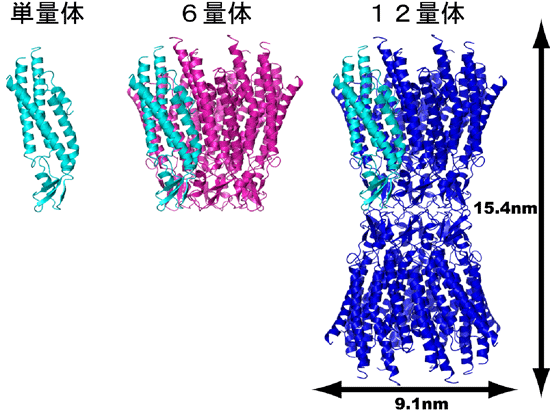 Fig. 1 Overall structure and formation process of connexin 26 gap junction channel.
Fig. 1 Overall structure and formation process of connexin 26 gap junction channel. From the left: connexin monomer, hexamer, and gap junction channel are shown in a ribbon diagram.
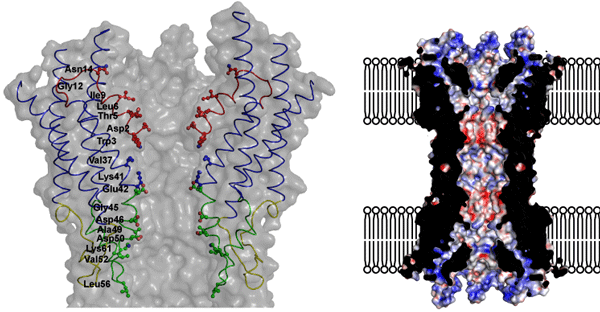 Fig. 2 Porous structure of gap junction channel.
Fig. 2 Porous structure of gap junction channel. The left figure shows the amino acid residue constituting a channel pore. The right figure shows the surface electric charges of the channel.
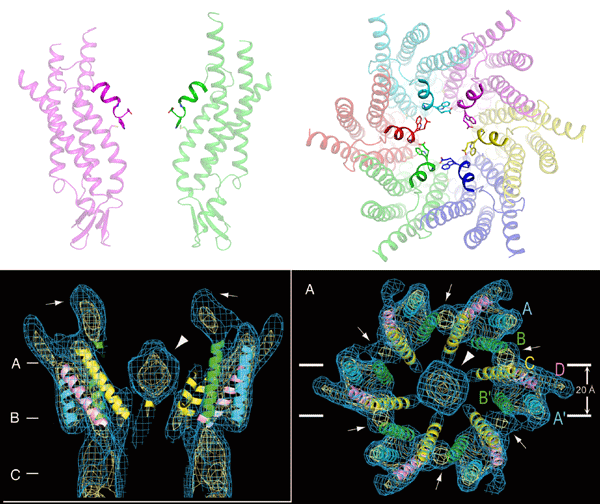 Fig. 3 Open (top) and closed (bottom) gap junction channels.
Fig. 3 Open (top) and closed (bottom) gap junction channels.No structure blocks the pore of the open channel. In contrast, a large block structure is observed at the upper portion of the pore of the closed channel.
The bottom figures are cited from Proc. Natl Acad. Sci. USA 104, 10034–10039 (2007).
<Glossary>
*1 Gap junction channel
An intercellular channel that directly connects cells. Gap junction channels allow the transport of molecules and ions with a weight of approximately 1000 or less, depending on their concentration gradient.
*2 X-ray crystal structure analysis
A method of analyzing the structure of a substance from its diffraction pattern and intensity, which are obtained by three-dimensionally arranging the substance into a regular pattern to form a crystal that is then irradiated with X-rays. It is frequently used for determining the structure of proteins.
*3 Cellular membrane
A membrane that separates the inside of a cell from the outside and is made of lipid phosphorous. The cellular membrane has a double-layer structure with the hydrophobic side of the lipid phosphorous molecules facing each other.
*4 Electron crystal structure analysis
A method of analyzing the structure of a substance from its diffraction pattern and intensity, which are obtained by two-dimensionally arranging the substance into a regular pattern and irradiating it with an electron beam. It is frequently used for determining the structure of membrane proteins.
|
For more information, please contact: Dr. Shoji Maeda (Osaka University) |
- Current article
- World's First Clarification of Three-Dimensional Structure of Human Gap Junction Channels - Hopes for contributing to establishment of strategies for medical treatment of deafness and irregular pulse (Press Release)
 ,
, .
.