First-Ever Observation of Electron State of Molecules in Aqueous Solution Using Soft X-Rays - Direct Observation of Change in Acetic-Acid Structure with pH Level (Press Release)
- Release Date
- 01 Oct, 2009
- BL17SU (RIKEN Coherent Soft X-ray Spectroscopy)
RIKEN
Japan Synchrotron Radiation Research Institute (JASRI)
Key research achievements
• The selective observation of molecules in aqueous solution by adjusting the energy of the irradiated soft X-rays to the absorption energy of acetic acid
• The establishment of an analysis method to distinguish the electron states of ionized and nonionized acetic acid molecules
• The detailed observation of the change in solute molecules in aqueous solution has become possible
|
RIKEN (Ryoji Noyori, President) succeeded in selectively observing, for the first time in the world, the electron state of molecules in aqueous solution at room temperature and ordinary pressure using the soft X-rays generated at SPring-8. This was achieved by a joint research team consisting of Shik Shin, team leader (also a professor at The Institute for Solid State Physics, The University of Tokyo), Yuka Horikawa, a junior research associate (JRA),*1 Takashi Tokushima, a research scientist, Yoshihisa Harada, a visiting scientist (also a research associate professor at the School of Engineering of The University of Tokyo), Osamu Takahashi (also an assistant professor at the Faculty of Science, Hiroshima University), and Chinani Ashish, a senior research scientist of the Excitation Order Research Team, Quantum Order Research Group at RIKEN SPring-8 Center (Tetsuya Ishikawa, Director); Yasunori Senba, a research scientist, and Haruhiko Ohashi, an associate chief scientist at JASRI; and Atsunari Hiratani, a professor at the Faculty of Science, Hiroshima University. An aqueous solution is a liquid consisting of substances dissolved in water; aqueous solutions exist in various forms around us, such as drinking water, seawater, and cell water. The substance dissolved in a liquid is called a solute, and the liquid is called a solvent in chemistry. Solute molecules are considered to move around the water solvent molecules and other solute molecules while interacting with them. Because the characteristics of a molecule are governed by the location of the atomic nucleus and the state of electrons that constitute the molecule, the effect of water molecules on solute molecules in aqueous solution, i.e., the mutual interaction between molecules, can be investigated in detail if the electron state of the solute molecule is observed. Such mutual interaction relates to not only chemical reactions occurring in the aqueous solution and physical properties, such as the viscosity of liquids, but also chemical reactions and protein structures in the cells of organisms; the clarification of the mutual interaction is expected to lead to the development of applied research in various fields. The joint research group confirmed that the selective observation of the electron state of molecules in an aqueous solution was possible using acetic acid,*2 which is familiar to us as a component of vinegar. When the pH of the aqueous solution*3 of acetic acid is changed from acidic to basic (alkaline), the structure of acetic acid changes owing to ionization.*4 The research group successfully demonstrated that the electron state is observable by measuring the change in the molecular structure. The research achievements were published in the online version (30 September; 1 October 2009 Japan time) and printed version of the UK scientific journal Physical Chemistry Chemical Physics, Vol. 11, Issue 39; an illustration symbolizing the achievements of this research made the cover of the issue. Publication: |
<Figure>
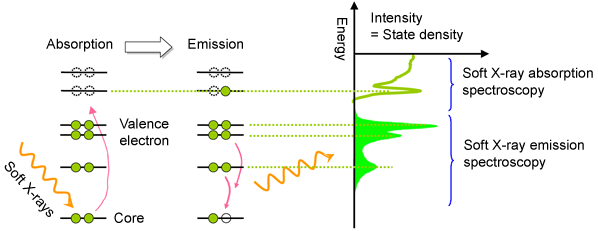 Fig. 1 Schematic of soft X-ray emission spectroscopy
Fig. 1 Schematic of soft X-ray emission spectroscopyThe lines represent molecular-orbit energy levels, on which filled and dotted circles indicate electrons and holes (the absence of an electron in an electron orbital), respectively. When soft X-rays with sufficiently high energy are irradiated onto a substance, an electron in the core receives its energy and jumps to an unoccupied molecular orbit (soft X-ray absorption), as shown in the left figure, or an electron in the core is expelled from the atom, and a hole is formed in the core. The hole is unstable and a valence electron involved in the bonding and reaction falls to the empty hole to achieve a stable condition within a very short duration of several femtoseconds (1 femtosecond = 10-15 s). Soft X-ray emission spectroscopy is a method of observing the X-ray emitted along with the transition of the electron. The state of the valence electrons is clarified by observing the intensity distribution of photon energy at the soft X-ray emission.
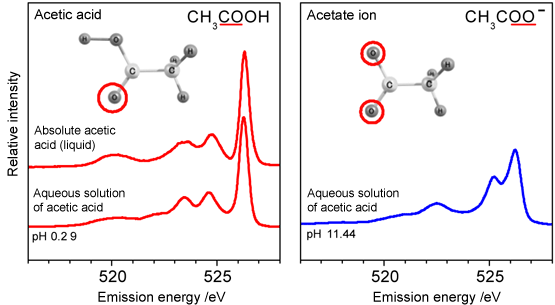
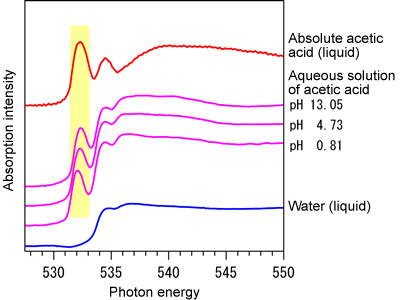 Fig. 2 Soft X-ray absorption spectra
Fig. 2 Soft X-ray absorption spectraThe red line, three pink lines, and blue line indicate the absorption spectra of absolute acetic acid, aqueous solutions of acetic acid with different pHs, and water. In the spectrum of absolute acetic acid, a characteristic peak is observed at about 532 eV. A similar peak is also observed in the spectrum of each aqueous solution of acetic acid (in the region colored in yellow). This peak is not observed in the spectrum of water and is thus considered to originate from acetic acid. By adjusting the energy of the irradiating soft X-rays to the light energy of this peak, the selective excitation of acetic acid is possible.
|
||||||
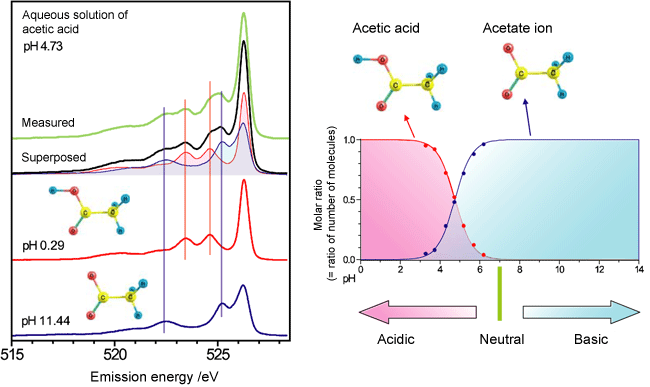
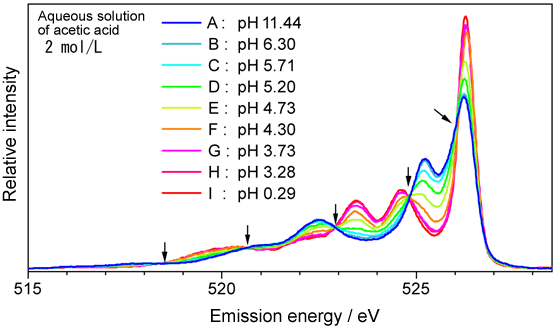 Fig. 4 Emission spectra of aqueous solution of acetic acid
Fig. 4 Emission spectra of aqueous solution of acetic acidThe shape of the spectrum changes with pH. However, there are points at which relative intensity is not changed regardless of pH, i.e., isoemission points, as indicated in the figure by black arrows. The isoemission points are observed when the spectrum comprises two components and only their proportions change owing to changes in conditions.
|
||||||
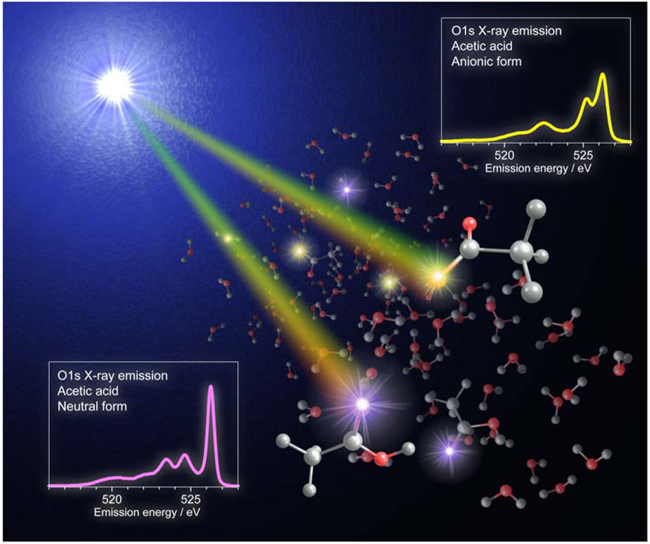 Fig. 6 Illustration based on this research achievement
Fig. 6 Illustration based on this research achievementThe illustration was drawn by Yuka Horikawa (JRA) and Takashi Tokushima (research scientist). It is the original of the illustration appearing on the cover of Physical Chemistry Chemical Physics, Vol. 11, Issue 39. It illustrates acetic acid molecules dispersed in water and the emission from the molecules upon the irradiation of soft X-rays from above the water surface.
<Glossary>
*1 Junior research associate (JRA)
The JRA system was started by RIKEN in 1996; RIKEN hires young researchers working toward a Ph.D. as part-time research scientists to promote cooperation between experienced researchers with extensive practical knowledge and research experience and young researchers with innovative ideas and enthusiasm.
*2 Acetic acid
Acetic acid is an ingredient of vinegar and a source of the sour taste and aroma of vinegar. The aqueous solution of acetic acid used in this study was prepared using high-purity acetic acid for laboratory use to minimize the effect of impurities. High-purity acetic acid is also called glacial acetic acid because it solidifies at temperatures lower than about 17oC.
*3 pH of aqueous solution
pH is used as a measure of the acidity or basicity (alkaline) of a solution. Pure water has a pH of 7 (neutral). When pH < 7, the solution is acidic, whereas when pH > 7, the solution is basic. The pH of aqueous acetic acid is about 5, and it is mildly acidic. Although the pH of solutions is generally in the range of 0-14, there is no limit to the value of pH in principle, because the pH is calculated from the concentration of hydrogen ions. pH may have a negative value for a highly concentrated acidic solution, and may exceed 14 for a highly concentrated alkaline solution.
*4 Ionization
Ionization is to form positive and negative ions when an atom or molecule of a material dissolves in a solvent. Salts such as sodium chloride, acids such as acetic acid and citric acid, and bases such as sodium hydroxide (caustic soda) are familiar examples of compounds that are ionized when they dissolve in water. Sodium chloride is separated into sodium ions and chloride ions. Acetic acid is separated into acetate ions and hydrogen ions; however, the rate of ionization depends on the pH of the solution because the tendency of acetic acid to ionize in water is weak.
|
For more information, please contact: or Dr. Takashi Tokushima (RIKEN) |
- Current article
- First-Ever Observation of Electron State of Molecules in Aqueous Solution Using Soft X-Rays - Direct Observation of Change in Acetic-Acid Structure with pH Level (Press Release)
 ,
, .
.