World's First Superlight Irradiating Protein Microcrystals - One-micrometer-wide beam was realized at the protein crystallography beamline for the first time in the world. (Press Release)
- Release Date
- 27 Nov, 2009
- Protein Crystallography
RIKEN
Key Research Achievements
• Enabling the analysis of 10-μm-diameter crystal structures that were difficult to analyze using conventional technologies
• Contribution to the structural analysis of key proteins involved in life phenomena and diseases
• Availability of 1-μm-diameter focused beams to SPring-8 users in fiscal year 2010
|
RIKEN (Ryoji Noyori, President) has succeeded in forming ultrahigh-brilliance microbeams of 1 μm diameter, which have the world's highest precision as a protein crystallography beamline, at SPring-8 for the first time in the world. This was achieved by the group of Masaki Yamamoto, Group Director of the Research Infrastructure Group at RIKEN SPring-8 Center (Tetsuya Ishikawa, Director). Conventional X-ray protein crystallography*1 requires crystal diameters of 20-30 μm. However, it was difficult to analyze key proteins involved in life phenomena and the development of diseases and disorders because many of them yield only 10-μm-diameter or smaller microcrystals; thus, conventional X-ray crystallography is difficult to apply to such proteins. In this research, scientists have succeeded in producing 1-μm-diameter microbeams with an ultrahigh brilliance sufficient for crystallography by combining a hybrid undulator to generate high-brilliance synchrotron radiation, an ultrahigh-precision spectrometer, and an X-ray mirror fabricated by elastic emission machining (EEM)*2 at RIKEN Targeted Proteins Beamline, BL32XU, in SPring-8. In the near future, technologies for controlling beams and equipment to collect measurement data obtained by irradiating beams to microcrystals will be introduced and the required adjustments will be made, with the aim of making the 1-μm-diameter beams available to SPring-8 users. In fiscal year 2010, the beamline for the structural analysis of protein microcrystals using the world's first 1-μm-diameter focused beams will be open to users. We hope that studies of proteins that were difficult to analyze by conventional crystallography will markedly advance. This research was carried out with the project theme of "Development of synchrotron beamlines dedicated to the measurement of micron-size protein crystals (development of high-brilliance microbeamline (Spring-8 beamline))" in the Targeted Proteins Research Program,*3 a research commission project supported by the Ministry of Education, Culture, Sports, Science and Technology. |
<Figure>
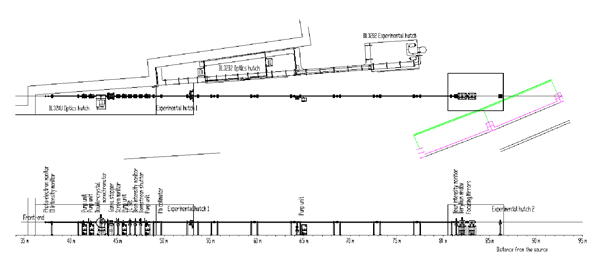
Fig. 2 Overall structure of RIKEN Targeted Proteins Beamline (BL32XU)
The beamline apparatuses are arranged as follows. From upstream: a hybrid undulator to generate high-brilliance synchrotron radiation, a front-end (FE) slit to mitigate thermal load from synchrotron radiation (undulator and front-end slit are not shown in the figure), a high-precision two-crystal spectrometer to monochromate white X-rays, a transport channel (TC) slit as a virtual light source, a Kirkpatrick-Baez (K-B) mirror system to focus beams, and a sample stage. These apparatuses are connected via a vacuum pipe, through which synchrotron radiation passes.
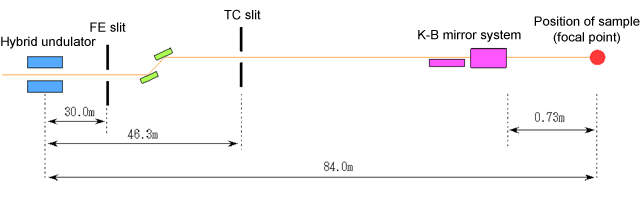
Fig. 3 Structure of optical focusing system to realize 1-μm-wide beam
The synchrotron radiation emitted from an inserted light source (insertion device (ID)) is narrowed to 0.3 mm width through a FE slit, then to 45 μm in the horizontal direction and 20 μm in the vertical direction through a TC slit, and the light is focused to form a 1-μm-wide beam after passing through vertical and horizontal focusing mirrors (K-B mirror system) placed immediately in front of the sample. To condense a large beam into a small one, it is necessary to increase the distance from the TC slit to the K-B mirror system and to decrease that from the K-B mirror system to the sample. Therefore, in this beamline, the entire length from the ID to the sample is approximately 85 m; this is longer than for the standard beamlines at SPring-8.
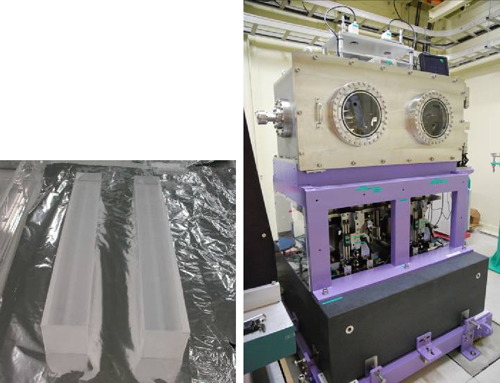
Fig. 4 Main body of ultrasmooth mirror to realize 1-μm-wide focused beam (left) and mirror adjustment system (right)
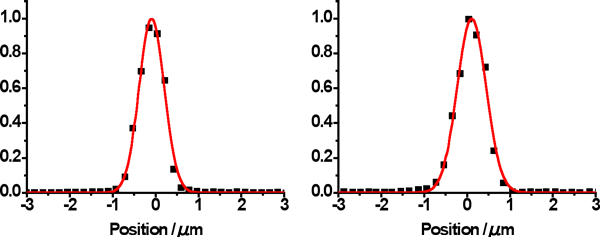
Fig. 5 Focused beam profiles in horizontal and vertical directions
Beam profiles were measured by inserting a gold wire in steps of 0.2 μm to the focal plane after an X-ray beam with a wavelength of 1 Å was focused to the position of the sample through the K-B mirror system (wire scanning). The half-widths of the focused beam were 0.69 and 0.78 μm in the horizontal and vertical directions, respectively. The beam intensity was 5.7 × 1010 photons/s.
<Glossary>
*1 Protein crystallography
Protein crystallography is an experimental method in which a protein, the final genetic product that carries out biological functions, is crystallized to determine its three-dimensional steric structure at the atomic level by X-ray diffraction. Currently, high-brilliance X-rays produced at synchrotron radiation facilities are generally used for protein crystallography.
*2 Elastic emission machining (EEM)
EEM is an ultrahigh-precision machining method using chemical reactions at the interface between microparticles and a workpiece. The surface of a workpiece can be machined without any mechanical load. The accurate supply of microparticles that are chemically reactive to the workpiece surface enables us to fabricate a smooth surface with atomic-level precision.
*3 Targeted Proteins Research Program
From among the proteins with structures that are extremely difficult to determine using the current level of technology, those important to academic research and industrial development are selected as targeted proteins. Targeted Proteins Research Program is a project supported by the Ministry of Education, Culture, Sports, Science and Technology with the following aims: (1) to develop technologies for analyzing the structure and functions of proteins that are difficult to analyze and (2) to clarify the structure and functions of targeted proteins. In research on technological development, the technologies for producing protein samples, analyzing protein steric structure, and controlling protein functions are the focus. In research on targeted proteins, the analyses of the structures and functions of targeted protein groups are being carried out to clarify fundamental life phenomena, contribute to medical fields and drug development, and apply findings to industries related to food and environment. These research studies are ongoing as an integrated project.
|
For more information, please contact: |
- Current article
- World's First Superlight Irradiating Protein Microcrystals - One-micrometer-wide beam was realized at the protein crystallography beamline for the first time in the world. (Press Release)

