Clarification of Structure of Metabolic Sensor "Band 3" in Red Blood Cells - V-Shaped Structures Alternately Opening Outward and Inward Similar to Ion Channels- (Press Release)
- Release Date
- 22 Jan, 2010
- electron microscope
RIKEN
Nagasaki International University
Key research achievements
• Immobilization of band 3 by placing its open end toward the outer surface of the membrane (opening outward) and structural analysis of two-dimensional crystals of band 3
• Discovery of three-dimensional structure of band 3, which is similar to that of ClC-type chloride ion channels
• Contribution to structural clarification of ion exchange transport mechanism of red blood cells
|
RIKEN (Ryoji Noyori, President) and Nagasaki International University (Yoshiko Shiotani, President) have succeeded in the three-dimensional structural analysis of a membrane transport protein,*2 "band 3,"*3 existing in large amounts in human red blood cells,*1 which carry oxygen. This was achieved through a joint research by Tomohiro Yamaguchi, a researcher, and Teruhisa Hirai, a team leader, of the Three-Dimensional Microscopy Research Team, Structural Physiology Research Group, RIKEN SPring-8 Center (Tetsuya Ishikawa, Director), Professor Naotaka Hamasaki of the Faculty of Pharmaceutical Science, Nagasaki International University, and Professor Dongchon Kang of the Faculty of Medical Sciences, Kyushu University. Band 3 is a membrane transport protein that exists in large amounts in the red blood cell membrane. It detects carbon dioxide dissolved in blood and promotes the release of oxygen from hemoglobin in red blood cells. This process is induced by the ion exchange transport mechanism, releasing oxygen intensively into tissues where active metabolism is taking place and regulating the supply of oxygen, which can be harmful in both excess and shortage, to appropriate amounts. Biochemical experiments on band 3 have been conducted over the years; however, because of the difficulty in the crystallization of band 3, there have been few reports on the analysis of the three-dimensional structure of band 3 since its rough structure observed by electron microscopy was reported in 1994. In this study, they attempted to stabilize band 3 by adding an ion transport inhibitor "H2DIDS,"*4 which immobilizes band 3 by covalent binding from outside the membrane. As a result, the two-dimensional crystallization*5 of band 3 significantly advanced. They succeeded in analyzing the three-dimensional structure of band 3 by electron microscopy, which enabled the observation of its crystals at a very low temperature (-269 °C) with a resolution of 7.5 Å, which has never been realized before. Moreover, they found that the three-dimensional structure of band 3 is similar to that of ClC-type chloride ion channels,*6 the structure of which has already been clarified. ClC-type chloride ion channels are membrane proteins that play an important role in muscle contraction. It is expected that the clarification of the structure of band 3 in this study will promote the structural clarification of the ion exchange transport mechanism of red blood cells. Moreover, the finding of a hitherto unknown similarity between the three-dimensional structure of band 3 and that of ClC-type chloride ion channels provides us important information about the classification of ion transporters and the evolution of the ion transport mechanism, which will contribute to the future progress of research in this field. This study was partially supported by a research fund for "Development of Fundamental Technologies for Structural Analysis of Proteins for Accelerating Drug Discovery" sponsored by the New Energy and Industrial Technology Development Organization (NEDO) and the result was published in the UK scientific journal Journal of Molecular Biology (online version). Publication: |
<Figure>
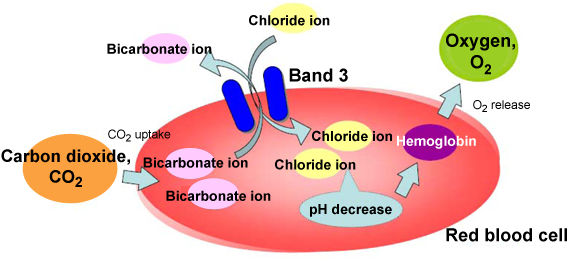
Fig. 1 Function of band 3
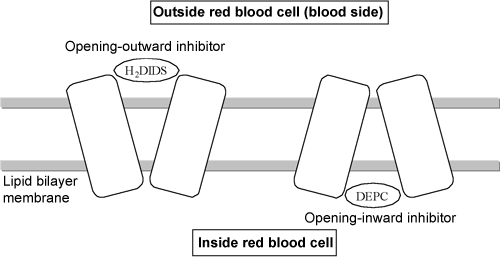
Fig. 2 Effects of inhibitors on band 3
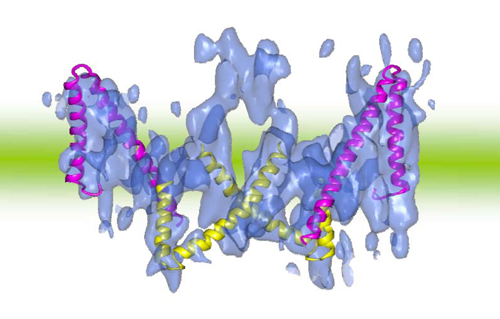
Fig. 3 Three-dimensional structure of band 3 (opening outward) and V-shaped structure of ClC-type chloride ion channels
<Glossary>
*1 Red blood cells
Blood is classified into the solid component (namely, blood cells) and the liquid component (namely, plasma). Blood cells are further classified into the following three components: red blood cells, white blood cells, and platelets. Red blood cells contain high levels of a protein called hemoglobin and are important because they carry oxygen from the lungs to all tissues of the body.
*2 Membrane transport protein
Among the proteins in biomembranes (membrane proteins), those that have the function of transporting substances are called membrane transport proteins. Some membrane transport proteins are channels that only allow the passage of transported substances along a gradient (i.e., the difference in concentration inside and outside the membrane). Others are transporters that actively transport substances using the energy of adenosine triphosphate (ATP) hydrolysis and the gradient of other transported substances. Channels can change their structures in order to open and close but merely act as pores through which substances pass. When membrane transport proteins act as transporters, they pump (transport) substances by changing their structures.
*3 Band 3
Band 3 is a membrane transport protein, which is also called anion exchanger 1 (AE1), existing in the human red blood cell membrane and renal collecting duct. Band 3 roughly consists of two domains: the hydrophilic domain existing on the cytoplasmic side and the membrane-spanning domain. The three-dimensional structure of the hydrophilic domain has already been clarified by X-ray structural analysis. The membrane-spanning domain (residues 361 to 911) analyzed in this study has more important functions because it carries out anion exchange transport. In the crystallization of band 3 in this study, the domain was bound to an inhibitor, H2DIDS, so that it was fixed open outward. A mutation of band 3 causes the onset of a hereditary disease, Southeast Asian ovalocytosis (SAO), which affects the shape of red blood cells, as well as the onset of distal renal tubular acidosis (dRTA).
*4 H2DIDS and DEPC
4,4'-Diisothiocyanatodihydrostilbene-2,2'-disulfonic acid (H2DIDS) and diethyl pyrocarbonate (DEPC) are inhibitors of band 3 ion transport. H2DIDS binds to band 3 in the cell membrane from outside the membrane, whereas DEPC binds to band 3 from inside the cell membrane. When H2DIDS binds to band 3, DEPC cannot bind to band 3, and vice versa. Therefore, it is considered that band 3 binds to these inhibitors with different structures (i.e., opening outward and opening inward).
*5 Two-dimensional crystallization
Membrane proteins are found in the lipid bilayer membrane. In two-dimensional crystals, membrane proteins are arranged regularly and two-dimensionally within the lipid bilayer membrane. Usually, the two-dimensional crystals are prepared by solubilizing the lipids and proteins separately, mixing them, and then removing the solubilizing agents by dialysis in order to reconstitute the lipid bilayer membrane. Images of the two-dimensional crystals are obtained by electron microscopy at various angles of inclination in order to collect three-dimensional data, which are then subjected to structural analysis. Because the membrane proteins are crystallized within the lipid bilayer membrane, the proteins can be observed under conditions closer to the physiological environment in two-dimensional crystallization than those in three-dimensional crystallization.
*6 ClC-type chloride ion channels
ClC-type chloride ion channels play an important role in muscles. The structure of a bacterium-derived homolog has already been clarified by X-ray structural analysis, that is, the homolog consists of seven duplexes. Some members of the ClC-type chloride ion channel family act not as channels but as transporters; the details of the transport mechanism of chloride ion channels have not been clarified. Dr. Roderick MacKinnon, who reported the three-dimensional structure of ClC-type chloride ion channels (on the basis of X-ray structural analysis), won the Nobel Prize in Chemistry for his research on cell membrane channels in 2003.
Red blood cells take up CO2 released by metabolically active cells into blood and convert the released CO2 into bicarbonate ions in the red blood cells. The exchange transport between these bicarbonate ions and chloride ions in blood carried out by band 3 significantly decreases the pH in red blood cells and promotes the release of oxygen from hemoglobin. By this mechanism, oxygen is intensively supplied to metabolically active tissues (which release large amounts of CO2).
It is considered that the structure of band 3 that alternately opens outward and inward enables the transport of ions. The inhibitor H2DIDS immobilizes band 3 so that it opens outward, whereas it is considered that DEPC immobilizes band 3 so that it opens inward.
Band 3 (blue) consists of a dimer of two closely-bonded molecules. One molecule has a pair of V-shaped structures that are arranged alternately opening outward and inward. These V-shaped structures agree well with the V-shaped structures of ClC-type chloride ion channels (helix models in red and yellow). Comparing the two amino acid sequences, homology was observed between the V-shaped structure of band 3 and that of ClC-type chloride ion channels.
For more information, please contact: |
- Previous Article
- Success in Highly Sensitive Visualization of Platinum Nanoparticles on Hair by Analysis Using High-Brilliance Synchrotron Radiation (Press Release)
- Current article
- Clarification of Structure of Metabolic Sensor "Band 3" in Red Blood Cells - V-Shaped Structures Alternately Opening Outward and Inward Similar to Ion Channels- (Press Release)
