Developing General-Purpose Plastic with Strength of Steel (Press Release)
- Release Date
- 19 Apr, 2010
- BL40B2 (Structural Biology II)
Japan Science and Technology Agency (JST)
Hiroshima University
|
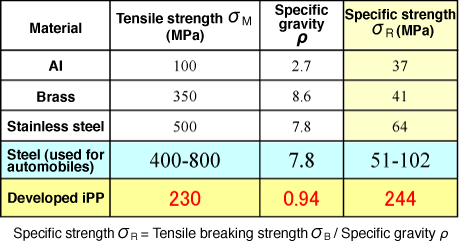
As part of Promoting Technology Transfer and Innovation by JST (Koichi Kitazawa, President), Masamichi Hikosaka, a specially appointed professor, and Kiyoka Okada, a doctoral researcher at the Graduate School of Integrated Arts and Sciences, Hiroshima University, have succeeded in developing a sheet-type recyclable ultrahigh-performance general-purpose polymer at a low cost*1 (general-purpose plastic) that has a specific strength*2 higher than that of steel and is light enough to float on water. Professor Hikosaka and his colleagues successfully increased the crystallinity of polypropylene, a typical general-purpose plastic, to nearly 100% by a unique crystallization method, in which a polymeric melt*3 is cooled below its melting point then elongated. The tensile strength of polypropylene was increased at least sevenfold to 230 MPa, and the specific strength became two- to fivefold higher than that of steel. Unlike expensive unrecyclable engineering plastics and fiber-reinforced plastics, ultrahigh-performance polymeric materials have additional advantages: they can be molded as cheaply and easily as conventional general-purpose plastics and can also be recycled. The achievement of this research was attributable to fundamental scientific advancements, i.e., clarification of the mechanism of polymer crystallization by Professor Hikosaka and his colleagues. In the future, it is hoped that the achievements of this research will contribute to establishing a low-carbon sustainable society based on energy and resource conservation at low cost by further developing this research and by promoting the use of the newly developed general-purpose plastics in Japan and overseas as an alternative to metals, ceramics, engineering plastics, and conventional general-purpose plastics, as well as steel plates used in automobiles and industrial materials. The research group aims at the practical application of the plastics in cooperation with companies participating in the joint research. The results of this research were published in the Japanese academic journal Polymer Journal in June 2010. Publication: |
<Figure>
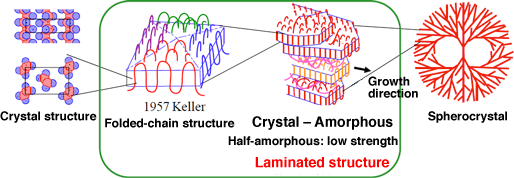
Fig. 1 Structure of conventional polymeric crystal
(Image courtesy of Professor Akihiko Toda at Hiroshima University)
Usually, long string-shaped polymers intertwine with each other in the melt (liquid) state, similarly to furballs. In the crystal state, polymers form a thin plate structure with a thickness of 10 nm and appear as if they are folded; this is called the folded-chain structure. Folded-chain structures and amorphous structures are alternately stacked to form laminated structures, from which a golf- ball-shaped rough crystal with a diameter of about 20 – 300 μm, called a spherocrystal, is formed. Therefore, the crystallinity of the spherocrystal is less than 50%, resulting in the low strength of the conventional plastic.
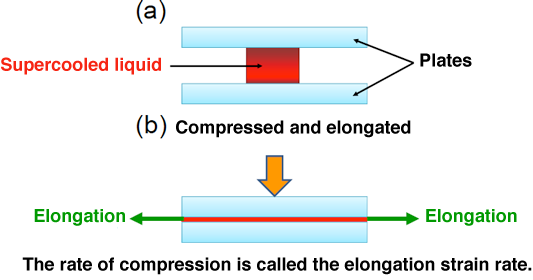
Fig. 2 Schematic of principle under elongational crystallization by compression
A polymeric melt is supercooled to start crystallization, and compressed from above to induce rapid elongation. Thus, elongational crystallization is achieved.
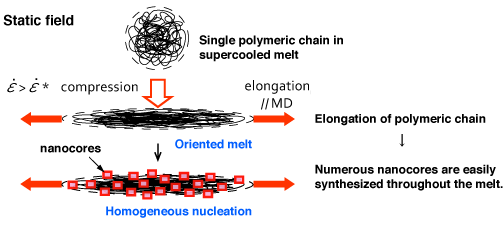
Fig. 3 Mechanism of synthesizing nano-oriented crystal (NOC)
When a supercooled polymeric melt is compressed, its polymeric chain is elongated, causing the melt to become oriented in one direction. Because of this, nanocores, which are the seeds of microcrystals, are rapidly synthesized throughout the melt.
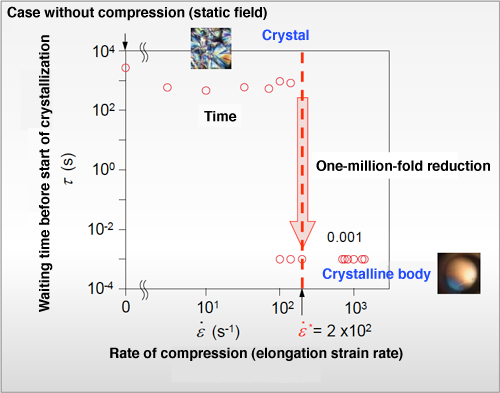
Fig. 4 Discovery of critical elongation strain rate
The waiting time required for the start of crystallization to occur (crystallization induction time) was previously about 103 s. This was reduced by a factor of a million to 10-3 s when a force sufficient to elongate a polymeric chain 200-fold in 1 s was applied during compression at the elongation strain rate indicated by the red dotted line. This is the critical elongation strain rate.
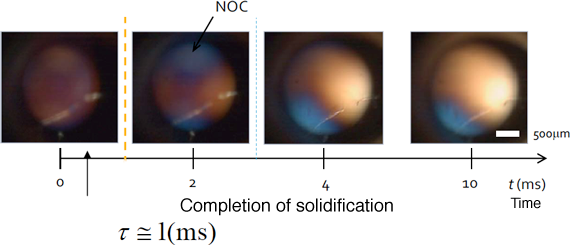
Fig. 5 Crystallization of NOC
These microscopic polarization images obtained using a high-speed camera show that NOC is crystallized instantaneously when the polymeric melt is compressed. The blue and yellow areas are evidence of oriented crystallization. The rapid oriented crystallization of NOC in only a few milliseconds was thus confirmed.
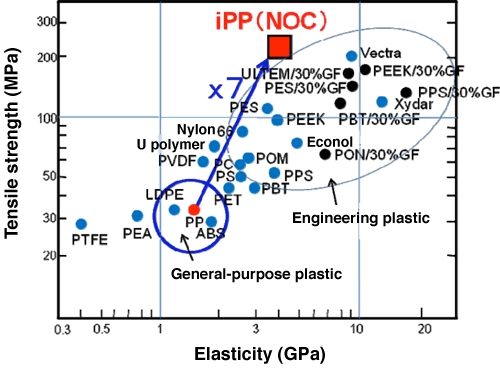
Fig. 6 Comparison of tensile breaking strength among plastic sheets
The tensile breaking strength of NOC sheets is about sevenfold higher than that of conventional general-purpose plastic sheets and equal to or higher than those of engineering and super engineering plastic sheets. NOC sheets exhibit the highest reported strength among conventional crystalline polymeric sheets.
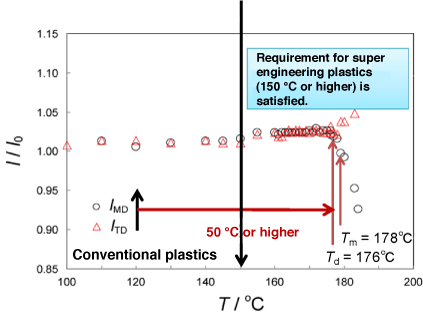
Fig. 8 High thermal resistance of NOC
The heatproof temperature is the temperature at which the degree of thermal deformation of a material becomes 3% or more. The heatproof temperature of NOC is at least 50 °C higher than that of conventional oriented polypropylene (OPP) and also surpasses that of general super engineering plastics, which have a heatproof temperature of 150 °C or higher.
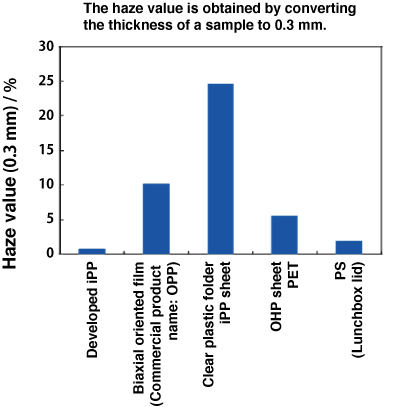
Fig. 9 Comparison of transparency among crystalline polymers
The Y-axis indicates the haze value (obtained by converting the thickness of a sample to 0.3 mm), which is an index of transparency. The leftmost bar indicates NOC, which has extremely high transparency. NOC is much more transparent than clear folders, considerably more transparent than polystyrene lunchbox lids, and as transparent as glass.
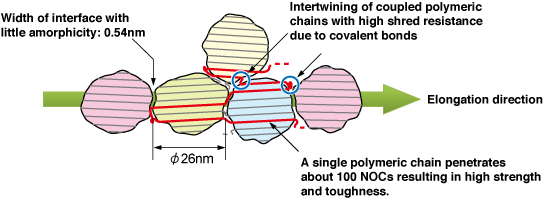
Fig. 10 Schematic of NOC armor model
NOCs are nanocrystals with a diameter of 20 – 30 nm and have an ordered arrangement in the elongation direction. Each strong string-shaped molecule is 2 μm long and binds about 100 NOCs by penetrating them. This structure was named the armor model because the string-shaped molecules, with a strength equivalent to that of diamond, combine almost all crystallized nanocrystals to form an armorlike structure.
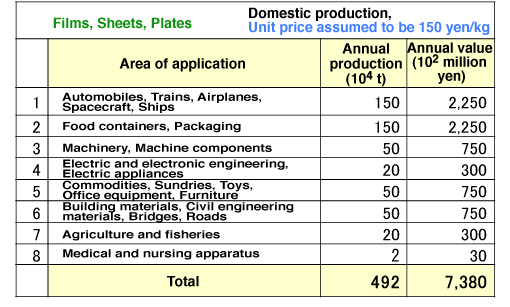
Fig. 11 Size of domestic market for ultrahigh-performance polymeric materials
Super engineering plastics are being used for films, sheets, and plates, and their domestic market amounts to over 700 billion yen. The world market is tenfold larger than the domestic market. These values indicate the potential market for NOC.
<Glossary>
*1 Low cost
Polypropylene and polyethylene are inexpensive and cost about 120-130 yen/kg.
*2 Specific strength
Specific strength is obtained by dividing the tensile breaking strength by the specific gravity. It is used for comparing the strength of different types of material.
*3 Melt
A melt is a liquid composed of one type of molten substance. A solution is composed of two or more types of molten substance. Liquids are classified into melts and solutions.
For more information, please contact: |
- Current article
- Developing General-Purpose Plastic with Strength of Steel (Press Release)