Better understand of NO-toxicity with synchrotron radiation (Press Release)
- Release Date
- 07 Dec, 2010
- BL09XU (Nuclear Resonant Scattering)
Japan Synchrotron Radiation Research Institute
|
Japan Synchrotron Radiation Research Institute (JASRI) (Shirakawa Tetsuhisa, President), in collaboration with the Massachusetts Institute of Technology (USA), the University of California, Davis (USA), have succeeded in characterizing the dinitrosyl iron complexes (DNICs) produced during breakdown of the iron-sulphur cluster in Rieske proteins by nitric oxide using the high-brilliance X-rays of SPring-8. They have discovered that Rieske proteins are susceptible to disassembly by NO, and that the predominant form of DNIC produced in these reactions is not the one containing a single iron atom as previously believed, but rather a dimeric form containing two atoms of iron. The precise structure of these DNICs is important to ascertain because it provides information about the mechanism of iron-sulphur cluster breakdown by nitric oxide. Such information can be used to learn more about possible treatments for NO-toxicity and to help design new and more powerful antimicrobial agents. These results were jointly obtained by S. J. Lippard, C. E. Tinberg, Z. J. Tonzetich, and L. H. Do of MIT, S. P. Cramerand H. Wang of UC Davis, and Y. Yoda (senior scientist) of JASRI. The research achievements will be published online in the American scientific journal, Journal of the American Chemical Society, on 6 December 2010. (Publication) |
<Background>
The transfer of electrons through proteins is a critical process carried out by all living organisms. Nature has evolved a host of specialized iron-sulphur proteins to accomplish this task. Each form of these so-called iron-sulphur clusters is designed to meet the specific needs of an organism. The destruction of proteins containing these iron-sulphur clusters constitutes a mechanism of toxicity and can lead to diseased states.
One such molecule capable of attacking the iron-sulphur components of electron-transfer proteins is nitric oxide (NO). This simple diatomic molecule is produced in nature during the immune response in many organisms to seek out and destroy invading pathogens. It is also produced during the high temperature combustion in cars or industrial apparatuses. The reaction of NO with iron-sulphur clusters leads to disassembly of the cluster and formation of various new iron compounds referred to as dinitrosyl iron complexes or DNICs.
The research groups at MIT, UC Davis and SPring-8 have utilized a synchrotron radiation based technique known as nuclear resonance vibrational spectroscopy (NRVS) to characterize the DNICs produced during breakdown of a specific class of iron-sulphur clusters by nitric oxide.
Synchrotron radiation is collimated, monochromatic and intense light with a wide variety of beam energy choice from a huge electron accelerator, such as the one in SPring-8. NRVS uses the extremely monochromatic X-ray at 14.4KeV, and probes the vibrational modes coupled with the 57Fe nuclear transition.
In comparison with traditional vibrational spectroscopy, such as FTIR and resonance Raman spectroscopy, it has several distinguished advantages. First, it is 57Fe selective. No signal arises from the background no matter how complicated the (protein) molecules. The site(s) of interest can also be selectively labeled with 57Fe; NRVS has different selection rules, and thus all the vibrational modes are detectable as long as the modes have 57Fe motion. In addition, both the intensity and energy can be simulated quantitatively, providing extra credence for the derived force constants and the related conclusion(s).
<Achievements>
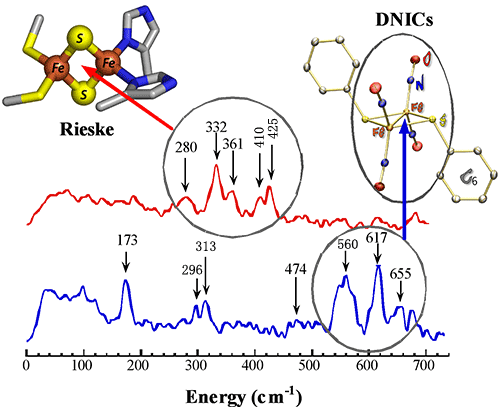
The Rieske protein has a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. Rieske proteins have been found in plants, animals, and bacteria. To examine the precise nature of the DNICs formed during reaction of a typical Rieske-type iron-sulphur protein, the NRVS spectra of ToMOC from Psuedomonas sp. OX,1, in both native and NO-treated forms were recorded and analyzed.
《Figure》
The native ToMOCox contains four well-resolved peaks in the region between 300 and 450 cm-1, comparable to the asymmetric and symmetric Fe–S stretching modes observed in the NRVS spectra of [2Fe-2S] ferredoxins. Upon treatment of ToMOCox with DEANO, which releases nitric oxide, the Fe–S modes disappear and three distinct new peaks are observed in the high-energy region at ~560, 617, and 655 cm-1. These peaks are attributed to the asymmetric and symmetric stretches of the N—Fe—N unit containing two nitric oxide ligands. The peak intensities and energies were also compared to those of several previously characterized one-, two-, and four-iron dinitrosyl compounds.
These results, together with EPR, Mössbauer, and UV-vis spectroscopic data, were used to determine that Rieske proteins are susceptible to disassembly by NO, and that the predominant form of DNIC produced in these reactions is not one containing a single iron atom, as previously believed, but rather a dinuclear form containing two atoms of iron.
The precise structure of these DNICs is important to ascertain because it provides information about the mechanism of iron-sulphur cluster breakdown by nitric oxide. Such information can be used to learn more about possible treatments for NO-toxicity and to help design new and more powerful antimicrobial agents.
<Future Developments>
This study revealed the nature of products produced during breakdown of Rieske-type iron-sulphur clusters by nitric oxide and shed some light on the mechanism by which this process occurs. In all instances during this study, an excess of NO was utilized to model conditions such as the immune response, where NO would be found in concentrations much greater than that of the iron-sulphur cluster containing protein. Future studies will examine the effect of other situations having smaller concentrations of NO on the ability of iron-sulphur cluster containing proteins to remain functional as electron transfer agents. Such work will also delve more deeply into the intricate mechanism of iron-sulphur cluster disassembly and may reveal the structures of additional intermediate species.
For more information, please contact: |
- Current article
- Better understand of NO-toxicity with synchrotron radiation (Press Release)