To What Extent Do Aerosols Cool The Earth? (Press Release)
- Release Date
- 30 May, 2011
- BL01B1 (XAFS)
Hiroshima University
High Energy Accelerator Research Organization
|
The research group of Yoshio Takahashi, Professor, and Takema Furukawa, a graduate student, of the Department of Earth and Planetary Systems Science, Graduate School of Science, Hiroshima University, found that the ability of organic aerosols*1 to absorb moisture in air and form clouds (cloud-forming ability) is lower than that previously estimated through experiments using facilities at the Photon Factory of the High Energy Accelerator Research Organization (KEK) and SPring-8. These achievements will contribute to the improvement of the accuracy in the estimation of the global cooling effects of aerosols, leading to the accurate prediction of global warming in the future. Publication: |
1. Background
Global warming is one of the serious global environmental issues confronting the world. To address global warming, it is important to accurately predict the changes in the trend of global warming in the future. According to the report of the Intergovernmental Panel on Climate Change (IPCC; winner of the 2007 Nobel Peace Prize), cloud formation by aerosol particles suspended in the air has global cooling effects.
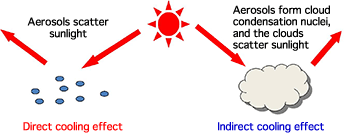
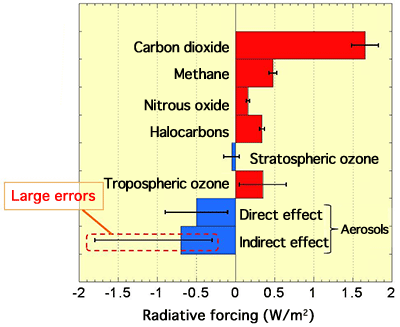
Because the aerosols suspended in air reflect and block sunlight, they have a direct global cooling effect (Fig. 1). Moreover, when the aerosols contain highly hygroscopic substances, they absorb the moisture in the air and become condensation nuclei that accelerate the formation of clouds. Because those clouds also reflect sunlight and cool the earth, the highly hygroscopic aerosols have an indirect global cooling effect (Fig. 1). In the IPCC report, these two types of cooling effect are considered separately and the negative contribution of each cooling effect to global warming is shown quantitatively. The indirect effect of cloud formation was reported to make a larger contribution than the direct effect. However, the report also acknowledged that there was a large uncertainty in the estimation of the global cooling effects of aerosols because of large errors resulting from the uncertainty in the composition and hygroscopicity of aerosols (Fig. 2). Therefore, it is necessary to clarify the detailed hygroscopicity and cloud forming ability of aerosols to improve the scientific reliability of their global cooling effects.
2. Research methods and achievements
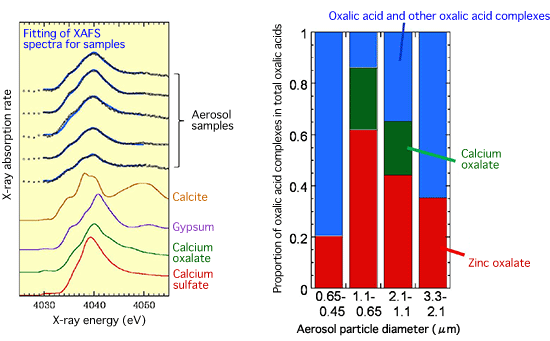
The substances that cause the indirect effect are roughly divided into two types, namely, inorganic aerosols such as sulfate aerosols and organic aerosols. Oxalic acid is the main component of organic aerosols. Through the analysis of X-ray absorption fine structure (XAFS)*2 spectroscopy at the Photon Factory of the Institute of Materials Structure Science, KEK (Tsukuba City, Ibaraki Prefecture), and the XAFS Beamline (BL01B1) of SPring-8 (Nishiharima, Hyogo Prefecture), Professor Takahashi and his colleagues found that most of the oxalic acid in the aerosols forms complexes*3 with metal ions such as calcium and zinc (Fig. 3). This finding is important in the following respects.
(1) When the oxalic acid forms complexes with calcium and zinc, its hygroscopicity is reduced to 1% or less compared with that of the oxalic acid that does not form complexes.
(2) When the hygroscopicity decreases, the ability of the oxalic acid to form cloud condensation nuclei also decreases.
(3) Therefore, the fact that the oxalic acid is present as complexes suggests that the indirect global cooling effect of the oxalic acid is not as large as previously estimated.
Their achievements were published in the May issue of the scientific journal of the European Geosciences Union Atmospheric Chemistry and Physics.
3. Future development
These results suggest that other dicarboxylic acids and organic acids may also form complexes, indicating the necessity of considering the complex formation with the coexisting metal ions in the discussion on the global cooling effects of the water-soluble organic aerosols.
These findings will contribute to the accurate prediction of global warming and the accurate quantification of the global cooling effects of aerosols by IPCC.
<<Glossary>>
*1 Organic aerosols
Particles suspended in the air such as yellow sand and tobacco smoke are called aerosols. Among them, those that consist of organic compounds are called organic aerosols.
*2 X-ray absorption fine structure (XAFS)
This is an experimental method of measuring the absorption spectra of substances while changing the energy of the X-ray. The chemical state and structure of the samples can be determined by analyzing the obtained spectra.
*3 Complexes
Complexes are compounds of nonmetal atoms bound to metal atoms. In this study, the complexes denote the compounds of oxalic acid (a nonmetal) and calcium and zinc (metals).
<<Figures>>
The graph is prepared on the basis of the data from the Fourth Assessment Report of IPCC (2007).
Radiative forcing: The red and blue bars indicate the contribution to warming and cooling, respectively.
spectroscopy and proportion of calcium oxalate complexes and
zinc oxalate complexes in total oxalic acids
Right: Plotted with particle diameter of aerosols
The aerosol samples were collected in Tsukuba City.
|
For more information, please contact: |
- Previous Article
- World’s First Realization of Soft X-ray Spectroscopic Experiments in Ultrahigh Magnetic Fields (Press Release)
- Current article
- To What Extent Do Aerosols Cool The Earth? (Press Release)