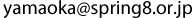New Model for Charge Transfer of Magnetic Organic Molecule, TDAE-C60
- Release Date
- 25 Oct, 2011
- BL17SU (RIKEN Coherent Soft X-ray Spectroscopy)
RIKEN
Key research achievements
• Photoelectron spectroscopy of α- tetrakis-dimethylamino-ethylene (TDAE)-C60 single crystal at SPring-8 and comparison of obtained result with theoretical one
• Transfer of one electron from TDAE to C60 induces magnetism in organic molecule, TDAE-C60
• Expected to be applied to high-density recording materials and magnetic compounds used as drugs
Scientists of RIKEN (President, Ryoji Noyori) have demonstrated the mechanism by which an organic molecule, originally without magnetism,*1 is magnetized, using a new model based on charge transfer. The achievements of this study were obtained by the following members: Hitoshi Yamaoka (Senior Research Scientist) and Yukiaki Ishida (Research Scientist, currently with The University of Tokyo), Masaharu Matsunami (Research Scientist, currently with the Institute for Molecular Science of National Institutes of Natural Sciences); Ritsuko Eguchi (Research Scientist, currently with the Faculty of Science, Okayama University) of RIKEN SPring-8 Center (Director, Tetsuya Ishikawa); Takashi Kambe (Associate Professor) of Faculty of Science, Okayama University (President, Kiyoshi Morita); Tohru Sato (Associate Professor) of Faculty of Engineering, Kyoto University (President, Hiroshi Matsumoto); Yasunori Senba (Research Scientist), and Haruhiko Ohashi (Associate Chief Scientist) of Japan Synchrotron Radiation Research Institute (President, Tetsuhisa Shirakawa). In general, compounds containing metal atoms or metals are magnetized. However, some nonmetal organic molecules are also magnetized. The research to clarify the mechanism behind this phenomenon has been intensively carried out. Typical magnetic organic molecules include TDAE-C60*4 in which an organic molecule, TDAE,*3 is bonded to a soccer-ball-shaped fullerene,*2 C60. It is considered that C60 is magnetized when an electron transfers from TDAE to C60. TDAE-C60 exhibits magnetism at very low temperatures of -257 oC or lower. This is based on the theory that C60 is stabilized when an electron transfers from TDAE to C60; as a result, electron spins of neighboring C60 molecules are aligned to induce strong magnetism. However, this theory has not been experimentally demonstrated owing to the limitation of the measurement method and no clear conclusion on the charge transfer has been obtained thus far. The research group carried out photoelectron spectroscopy*5 of an organic molecular magnet, a TDAE-C60 single crystal, using the soft RIKEN coherent soft X-ray spectroscopy beamline (BL17SU) at SPring-8. The results indicated that one electron transfers from TDAE to C60, as was also confirmed by theoretical calculation. On the basis of this result, the group proposed that the transfer of one electron causes the magnetism of TDAE-C60. In general, organic molecules are magnetized when isolated electron spins that can serve as a source of magnetism are formed as a result of charge transfer. Therefore, when a molecule that easily releases electrons and a molecule that easily accepts electrons are bonded, charge transfer occurs and the resultant compound tends to be magnetized. We may be able to freely design organic molecular magnetic bodies for use as magnets when the precise model of charge transfer proposed in this study is used, providing an effective direction toward magnet design. This achievement is expected to be applied to high-density recording materials used in next-generation memories and magnetic compounds used as drugs. The results were published online in the American scientific journal Physical Review B Rapid Communication. Publication: |
<<Glossary>>
*1 Magnetism
Magnetism is induced when the spins of electrons are aligned in a material. In particular, iron, nickel, and cobalt, all of which are 3d transition metals, are well known as easily becoming magnetic. The cases in which electron spins are aligned parallel and antiparallel are referred to as ferromagnetic (permanent magnet) and antiferromagnetic.
*2 Fullerene
Fullerenes are a material consisting of many carbon (C) atoms, and are also found in space. C60 is a spherical molecule with a soccer-ball shape consisting of 60 carbon atoms. Fullerenes are highly stable and have been found to exhibit various properties upon introducing other atoms inside and other molecules outside the C60 ball.
*3 Tetrakis-dimethylamino-ethylene (TDAE)
TDAE has the chemical formula C10H24N4. There are four N atoms surrounded by eight CH3 molecules. Its rational formula is [(CH3)2N]2C=C[N(CH3)2]2. TDAE has a high reducing power and reduces oxygen molecules in air. When it is oxidized by oxygen molecules, TDAE emits blue light.
*4 TDAE-C60
There are two types of TDAE-C60 : α-TDAE-C60 and α'-TDAE-C60. For α-TDAE-C60, structural phase transition is induced at approximately -103 oC and becomes ferromagnetic at approximately -257 oC. It has been considered that powder α'-TDAE-C60 is not magnetized at temperatures higher than approximately -271.5 oC; however, it has been found recently that an antiferromagnetic transition of α'-TDAE-C60 single crystals is induced at approximately -266 oC. Through heat treatment at 70 oC for approximately three hours, the α' phase changes into the α phase.
*5 Photoelectron spectroscopy
When X-rays are irradiated onto a sample material, electrons on the sample surface receive energy from the X-rays and are emitted from the surface with energy equivalent to the X-ray energy minus the binding energy. Photoelectron spectroscopy is a measurement method for examining the electronic state from the relationship between the number of electrons emitted from the sample surface and the energy. This technique enables the direct observation of the distribution of electron energy inside a material.
<<Figures>>
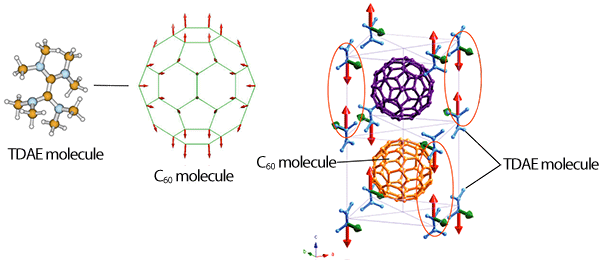
Left: Because TDAE is a strong electron supplier, an electron transfers from TDAE to C60, which slightly deforms the C60. The transferred electron is located near the equatorial region of C60.
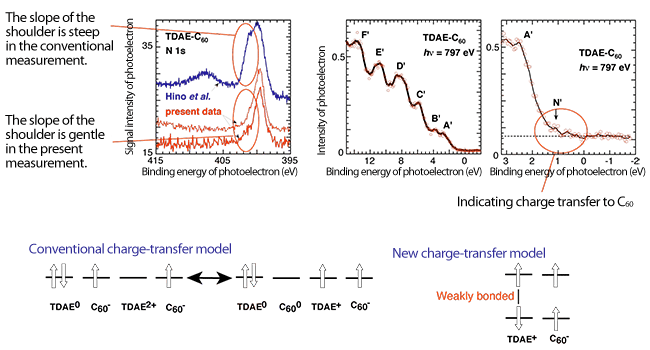
Right: The TDAE molecules of the TDAE-C60 crystals within the red circles weakly bond to each other as a result of the shift of TDAE molecules at low temperatures of -257 oC or lower.
The upper three graphs indicate the photoelectron spectra. The spectrum corresponding to nitrogen (N) in TDAE is significantly different from that obtained in the previous study. The charge-transfer model based on the photoelectron spectra previously obtained is shown at the lower left. A new model devised from the new result and theoretical calculation is shown at the lower right. In the new model, one electron transfers from TDAE to C60, and the neighboring TDAEs, the spin directions of which are opposite, are weakly bonded.
|
For more information, please contact: |
- Previous Article
- Clarifying the Bonding State of Aluminum Storing a Significant Amount of Hydrogen Using Synchrotron Radiation (Press Release)
- Current article
- New Model for Charge Transfer of Magnetic Organic Molecule, TDAE-C60