Elucidation of the Origin of High Oxygen Permeability in Praseodymium-Nickel Oxides (Press Release)
- Release Date
- 19 Oct, 2012
- BL02B2 (Powder Diffraction)
Tokyo Institute of Technology
Kyushu University
High Energy Accelerator Research Organization (KEK)
Tohoku University
Main points of the research
• Gallium and copper doping to praseodymium-nickel oxides is known to have the effect of enhancing oxygen permeability. This research elucidated the mechanism underlying the phenomenon, as well as giving an account of the mechanism that produces a large number of mobile oxygen atoms within the doped oxides.
• Identification of the oxygen permeability controlling parameter: i.e. the nuclear density*1.
• The results are expected to contribute to the development of new ion conductors and performance enhancement of solid oxide fuel cells.
|
Prof. Masatomo Yashima (Graduate School of Science and Engineering, Tokyo Institute of Technology), Prof. Tatsumi Ishihara (International Institute for Carbon-Neutral Energy Research/School of Engineering, Kyushu University) and other researchers conducted joint research on gallium and copper containing praseodymium-nickel oxide, and elucidated the mechanism that endows it with high oxygen permeability. This oxide compound is expected to be especially useful as a material for fuel cells and oxygen permeating membranes. Detailed examination of the crystal structure (atomic arrangement) of this oxide, using such techniques as neutron diffraction and synchrotron radiation X-ray diffraction, revealed that a large amount of redundant oxygen atoms are located in interstitial positions. The driving force that brings this configuration into existence was also determined: gallium assumes the role of introducing a large number of oxygen atoms into interstices, and copper provides the interstitial oxygen atoms with higher mobility. It was confirmed that, with a rise in temperature, the density distributions of lattice oxygen and interstitial oxygen become connected, facilitating oxide ion migration. The associated nucleus density was also verified to increase in sync with a rise in oxygen permeability. The research results can provide a new concept for the design of ion conducting materials with enhanced oxygen permeability, and possibly lead to the development of a new breed of them. Highly oxygen-permeable ion conductors can incorporate atmospheric oxygen more efficiently, promising increased performance of solid oxide fuel cells*2, as well as accelerating research and development. These results appeared in the online version of Chemistry of Materials (an America Chemical Society publication) on the 10th of October. Publication: |
<<Figures>>
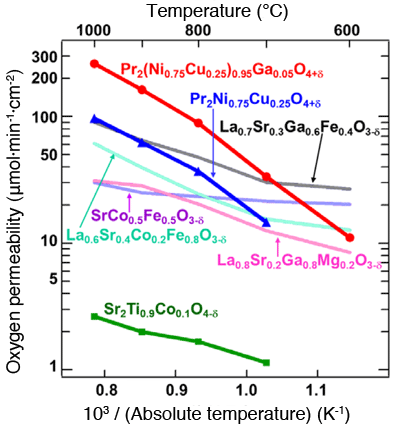
in various mixed conductors*3.
In contrast to the fact that the oxygen permeability of Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ( ) is higher than those of conventional mixed oxides, that of Sr2Ti0.9Co0.1O4-ε(
) is higher than those of conventional mixed oxides, that of Sr2Ti0.9Co0.1O4-ε( ) is extremely low in spite of its having the same K2NiF4-type structure*4.
) is extremely low in spite of its having the same K2NiF4-type structure*4.
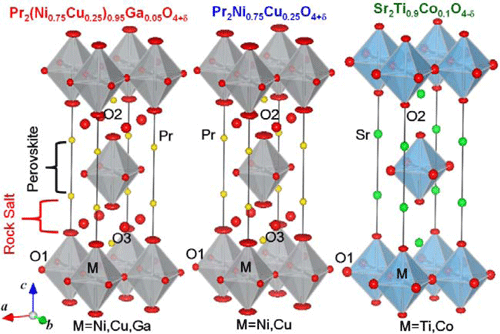
- Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ, Pr2Ni0.75Cu0.25O4+δ and Sr2Ti0.9Co0.1O4-ε
- as revealed through structural analysis based on neutron diffraction data.
All these have K2NiF4-type structure. In case of gallium and copper doped crystals, i.e. Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ and Pr2Ni0.75Cu0.25O4+δ, redundant O3 is visibly incorporated within the crystal lattice.
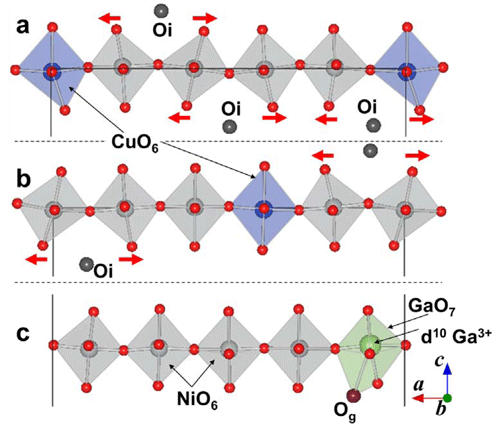
by first-principle calculation*5.
The cell structure of Pr40Ni15Cu4GaO86 is an approximate supercell of Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ. As the top (a) and middle (b) figures show, the apical oxygen located near an interstitial oxygen Oi shifts (misaligned) in the opposite direction from it and maintains a certain distance (red arrow). Local relaxation is observed in the vicinity of d10 Ga3+ dopant, as shown in the bottom figure (c), which has the effect of stabilizing differently-positioned interstitial oxygen Og.
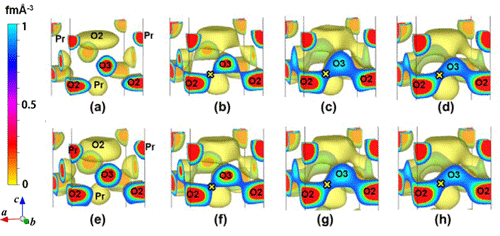
Pr2Ni0.75Cu0.25O4+δ, and (e-h): those of Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ.
O2 represents apical oxygen, and O3 interstitial oxygen. x identifies the minimum nuclear-density bottleneck in a diffusion path connecting O2 and O3. (a) 25°C, (b) 602°C, (c) 807°C, (d) 1011°C, (e) 20°C, (f) 605°C, (g) 810°C, (h) 1011°C.
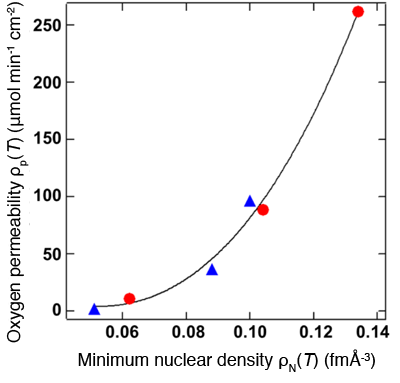
in minimum nuclear density ρN(T).
<<Glossary>>
*1 Nuclear density
The density associated with a nucleus. A crystal consists of atoms, and the atoms in turn consist of a nucleus and electrons. The spatial distribution of nuclear density can be ascertained by analyzing neutron diffraction data (the neutron, with no charge, is diffracted by nuclei).
*2 Solid-oxide fuel cell (SOFC)
A type of fuel cell that uses solid oxides as its electrolyte. Owing to its high operating temperature (400 - 600°C), it is expected to deliver higher generation efficiency than the polymer electrolyte fuel cell (PEFC).
*3 Mixed conductor
A solid or liquid that delivers electric conductivity by means of two or more charge carriers (e.g. ions and electrons) is called a mixed conductor. The target materials of this study - Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ, Pr2Ni0.75Cu0.25O4+δ and Sr2Ti0.9Co0.1O4-ε - are mixed conductors with two charge carriers, i.e. an oxide ion (O2-) and an electron hole (positive hole).
*4 K2NiF4-type structure
A crystal structure characterized by layer-by-layer stacking of two distinct structures: a perovskite structure, which consists of an octahedron of oxygen atoms with a dissimilar atom (e.g. transition metal ion) at the center, and a rock-solid structure (Fig. 2).
*5 First-principle calculation
A method to study the structure and properties of matter through calculations based solely on quantum mechanical principles. In this study, the first principle calculation approach was applied to confirm that an atom can stay stably in an interstitial position, as well as to examine localized structures and electron density distributions.
|
For more information, please contact:
Prof. Tatsumi Ishihara (International Institute for Carbon-Neutral Energy Research, Kyushu University)
HIGH ENERGY ACCELERATOR RESEARCH ORGANIZATION, KEK
Assistant Prof. Kenji Ohoyama (Institute for Materials Research, Tohoku University) |
- Previous Article
- New Possibilities for LED Illumination: The Development of New Phosphor from Commonplace Elements (Press Release)
- Current article
- Elucidation of the Origin of High Oxygen Permeability in Praseodymium-Nickel Oxides (Press Release)