Structure of the trypanosome alternative oxidase in complex with a potent inhibitor, a promising drug candidate for the treatment of African sleeping sickness (Press Release)
- Release Date
- 05 Mar, 2013
- BL41XU (Structural Biology I)
- BL44XU (Macromolecular Assemblies)
The University of Tokyo
Kyoto Institute of Technology
|
A joint research group of Profs. K. Kita (the University of Tokyo) and S. Harada (Kyoto Institute of Technology) succeeded in X-ray structure analysis of the trypanosome alternative oxidase (TAO) using X-rays produced by SPring-8 and Photon Factory. TAO is a promising drug target for the treatment of African sleeping sickness caused by Trypanosoma brucei. Structural information derived from the current structure of TAO in complex with a potent inhibitor that can cure infected goats and mice will accelerate the design of better therapeutic agents for African sleeping sickness, and it represents that results of basic science carried out in Japan will contribute to the improvement of health and economy of developing counties. Publication: |
<<Figures>>
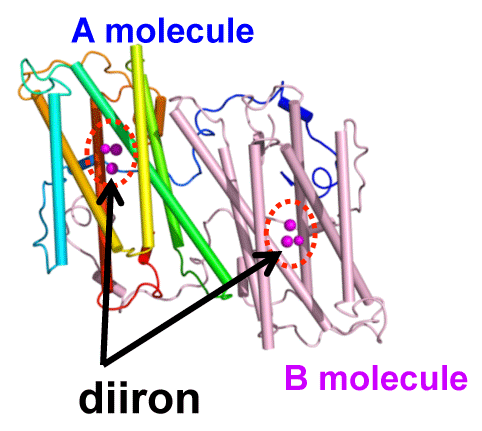
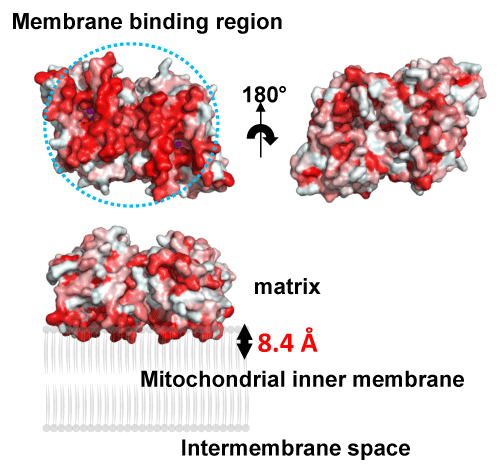
proposed binding model of the TAO dimer to membranes.
The red color highlights highly hydrophobic areas.
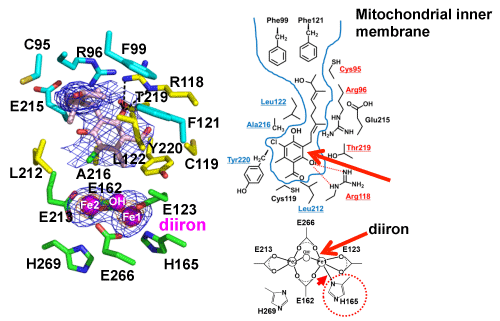
An inhibitor binds to a hydrophobic pocket near diiron.
|
For more information, please contact: Prof. Shigeharu Harada (Kyoto Institute of Technology) |
- Previous Article
- High-Accuracy Magnetic Property Measurement Method by Separating Spin and Orbital Magnetic Moments (Press Release)
- Current article
- Structure of the trypanosome alternative oxidase in complex with a potent inhibitor, a promising drug candidate for the treatment of African sleeping sickness (Press Release)