Unconventional Glass Structure Is Key to Achieving Ultrahigh Refractive Index (Press Release)
- Release Date
- 03 Aug, 2013
- BL04B2 (High Energy X-ray Diffraction)
Institute of Industrial Science, The University of Tokyo
Key points of this research
♦ Successful development of two types of colorless and transparent glass with refractive index higher than 2.2 and discovery of unconventional and strange glass structure as the key to achieving such a high refractive index.
♦ Clarification of factors that promote vitrification and the causes of the high refractive index at the atomic level for the first time in the world by the three-dimensional visualization of the atomic arrangement in glasses.
♦ Development of glasses with excellent optical properties for use as optical lenses with ultrahigh definition and high resolution.
|
Glasses with a high refractive index of 1.8 or above are mainly used as lenses. However, there seems to be a major theoretical hurdle in the development of colorless and transparent glasses with high refractive indices. A research group led by Atsunobu Masuno (assistant professor) and Hiroyuki Inoue (professor) of the Institute of Industrial Science, the University of Tokyo, Shinji Kohara (senior scientist) of the Japan Synchrotron Radiation Research Institute (JASRI), Alex C. Hannon (research scientist) of the Rutherford Appleton Laboratory in the United Kingdom, and Eugene Bychkov (professor) of the Université du Littoral in France succeeded in developing two types of new glass composed of only a rare-earth oxide (La2O3) and a niobium oxide (Nb2O5) (Fig. 2) by containerless processing*1 (Fig. 1). Previously, these oxides had not been considered as components of glasses. Of the two newly developed glasses, one was rich in La2O3 and the other was rich in Nb2O5. Both were colorless and transparent and had extremely high refractive indices ranging from 2.1 to 2.2. This international joint research team conducted a structural analysis through experiments involving high-energy X-ray diffraction*2 and neutron diffraction*3 as well as computer simulations. As a result, they found that the direct causes of the high refractive index of the developed glasses were the very high ionicity and the densely packed state of the elements contained in the glasses. Also, they clarified that the completely different local atomic-level structure from that of common glasses is the cause of the high density of the elements. Their achievements were not merely the development of glasses with new compositions. They demonstrated that glasses can be synthesized from combinations of elements that previously had not been considered as glass components in the field of glass science. They also demonstrated that such glasses exhibit excellent properties by atomic-level analysis. The results of this research may contribute to a significant expansion of the framework of glass science. Also, these achievements are expected to lead to the development of products such as ultrahigh-definition and high-resolution cameras for cell phones and tablet PCs and compact lenses for endoscopes in the near future. Publication: |
<<Figures>>
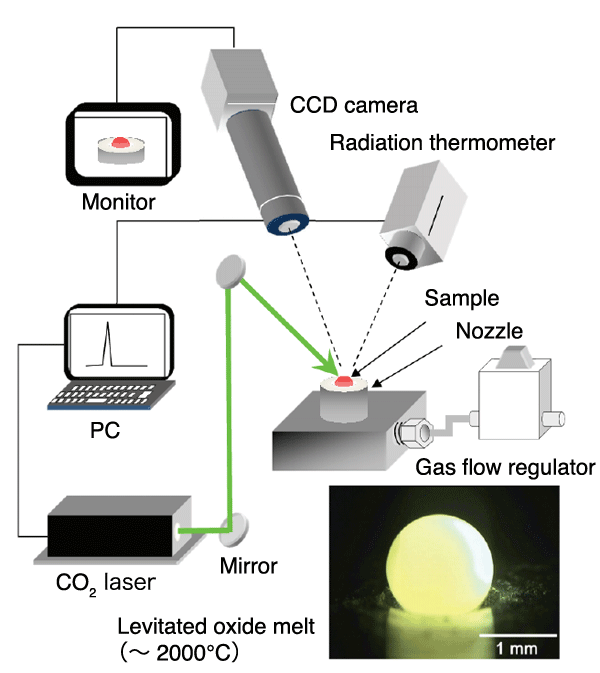
A material is kept levitated by gas ejected from a conical nozzle and is melted by heating with a CO2 laser. The photograph shows an oxide melt (glass bead melt) levitated at 2000°C.
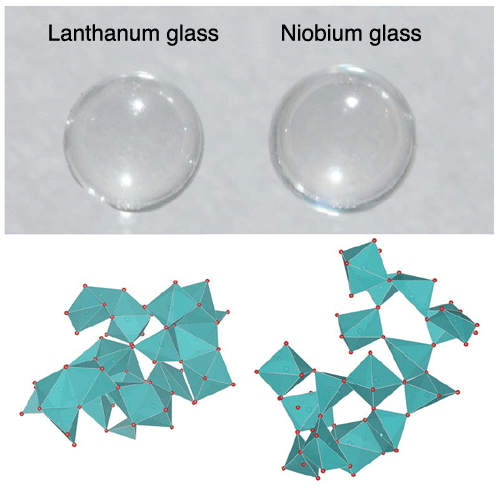
niobium glass (Nb2O5-rich) synthesized by containerless processing
Although the two glasses in the photograph appear to be the same colorless and transparent glass, there is a significant difference in their three-dimensional atomic arrangements, determined by computer simulations on the basis of experimental data.
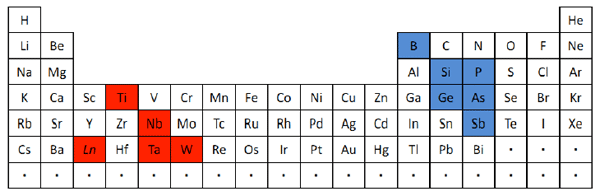
Common oxide glasses must contain one of the elements indicated in blue in the upper right of this periodic table. On the other hand, the results of this research have shown that glasses can also be synthesized from combinations of only the elements in red in the lower left of the table.
<<Glossary>>
*1 Containerless processing
In general methods of glass synthesis, the main factor preventing vitrification and promoting crystallization is crystal nucleation on the walls of containers. In containerless processing, materials are levitated in air during synthesis so that crystal nucleation on the walls is virtually eliminated. Elements that are less likely to be vitrified by general methods can be easily vitrified by this method. A gas-jet levitation furnace (Fig. 1) was used to provide the containerless condition in this research. The material was kept levitated by vertically injecting gas from a conical nozzle at the bottom of the furnace and was melted using a CO2 laser.
*2 High-energy X-ray diffraction
When X-rays, which are electromagnetic waves, are incident on atoms arranged according to a specific rule in a material, waves scattered from each atom interfere with each other to produce high intensity diffracted waves that propagate in only a certain direction (diffracted X-rays). This phenomenon is called X-ray diffraction and is used to determine the atomic arrangement of materials. In particular, the X-ray diffraction carried out at SPring-8, the world’s largest synchrotron radiation facility, located in Harima Science Park in Hyogo Prefecture, is called high-energy X-ray diffraction because the facility provides high-energy X-rays with high penetrating power.
*3 Neutron diffraction
The mechanism of neutron diffraction is the same as that of X-ray diffraction. The atomic arrangement of materials can also be determined using neutrons. However, while X-rays are scattered by electrons in atoms, neutron beams are scattered by nuclei, resulting in differences in the sensitivity of detection depending on the constituent atoms. X-ray diffraction and neutron diffraction, therefore, can provide different information on the same material. Recently, X-ray diffraction and neutron diffraction have been frequently used in a complementary manner.。
|
For more information, please contact: |
- Previous Article
- World’s first observation of hollow atom creation by successive X-ray hits (Press Release)
- Current article
- Unconventional Glass Structure Is Key to Achieving Ultrahigh Refractive Index (Press Release)