Successful Development of High-Energy-Density, High-Safety, and Low-Cost Rechargeable Batteries – From lithium to magnesium metal - (Press Release)
- Release Date
- 12 Jul, 2014
- BL01B1 (XAFS)
- BL02B2 (Powder Diffraction)
- BL14B2 (Engineering Science Research II)
Kyoto University
Japan Science and Technology Agency
Japan Synchrotron Radiation Research Institute (JASRI)
|
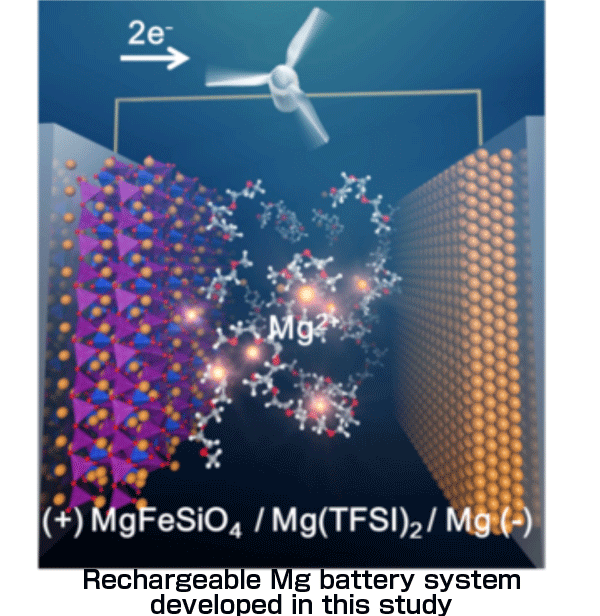
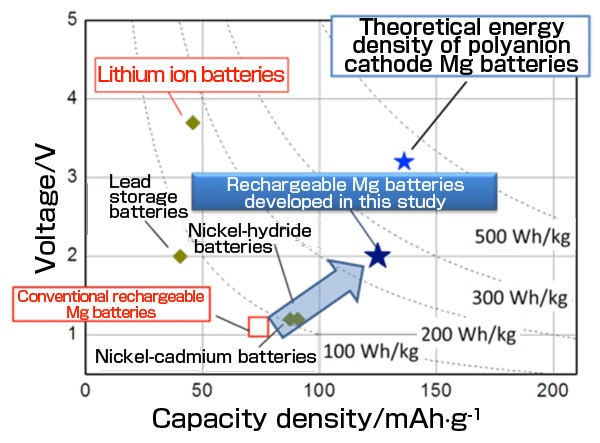
A research group led by Yoshiharu Uchimoto (professor) and Yuki Orikasa (assistant professor) of the Graduate School of Human and Environmental Studies, Hiroshi Kageyama (professor) of the Graduate School of Engineering, and Cédric Tassel (program-specific assistant professor) of the Hakubi Center for Advanced Research, Kyoto University, in collaboration with JASRI, succeeded in developing high-energy-density rechargeable magnesium (Mg) batteries that can replace conventional lithium ion batteries. The newly developed rechargeable batteries will be produced at a lower cost because they mainly consist of Mg, iron (Fe), and silicon, which are abundant resources. Also, the thermal stability of the batteries is improved by using Mg metal with a high melting point, resulting in marked improvements in their safety compared with that of conventional lithium ion batteries. Their achievements were published in the British online journal Scientific Reports published by Nature Publishing Group on 11 July 2014. This study was carried out as part of the “Innovation of s-Block Metal Batteries towards Low-Carbon Societies” project (research director, Professor Yoshiharu Uchimoto of the Graduate School of Human and Environmental Studies, Kyoto University; research period, from October 2008 to March 2014) within the research scope of the “Creation of Innovative Technologies to Control Carbon Dioxide Emissions” project [research supervisor: Itaru Yasui, President of the National Institute of Technology and Evaluation (NITE)] of the Core Research for Evolutional Science and Technology (CREST) supported by the Japan Science and Technology Agency (JST). Because of the high theoretical capacity density, abundant resources and high safety, rechargeable Mg batteries*1 are expected to be put into practical use as rechargeable batteries with performance exceeding that of lithium ion batteries*2. The problems, however, are the extremely slow electrode reactions due to divalent Mg ions having a stronger ionic interaction and a greater difficulty in diffusing through a solid phase than monovalent lithium ions. Also, no Mg electrolyte solution for stable and safe charge-discharge reactions that enable repeated deposition and dissolution of Mg metal has yet been found. Namely, it is necessary to resolve the issues concerning the cathode and the electrolyte solution in order to fabricate rechargeable Mg batteries. The research group reported on a MgFeSiO4 cathode material, in which the diffusion pathway for Mg ions is maintained by accurately controlling the crystal structure of the cathode material. Using this material, twice the number of Mg ions can be inserted and extracted, compared with conventional cathode materials. Because this material is stabilized by Si-O bonds, it enables repeated charge-discharge reactions over a long period of time. Also, the research group demonstrated the stable operation of the Mg metal anode using an electrolyte composed of Mg bis(trifluoromethylsulfonyl)imide [Mg(TFSI)2] and triglyme. The mechanism of stable and high-energy-density charge-discharge reactions was clarified using the high-brilliance synchrotron radiation at SPring-8*3. Combining the above cathode and electrolyte with an anode made of Mg metal, the research group fabricated rechargeable Mg batteries that offer the world’s highest performance. The results of this research and development will promote the practical use of rechargeable Mg batteries. The realization of a low-cost and safe electric energy storage medium will enable the storage of renewable energy, the supply of which fluctuates greatly under existing conditions, thus opening new possibilities of stable energy supply. Publication |
《Figures》
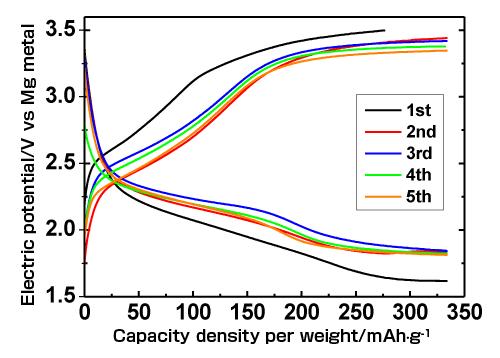
These profiles demonstrate the possibility of achieving a stable and high capacity density.
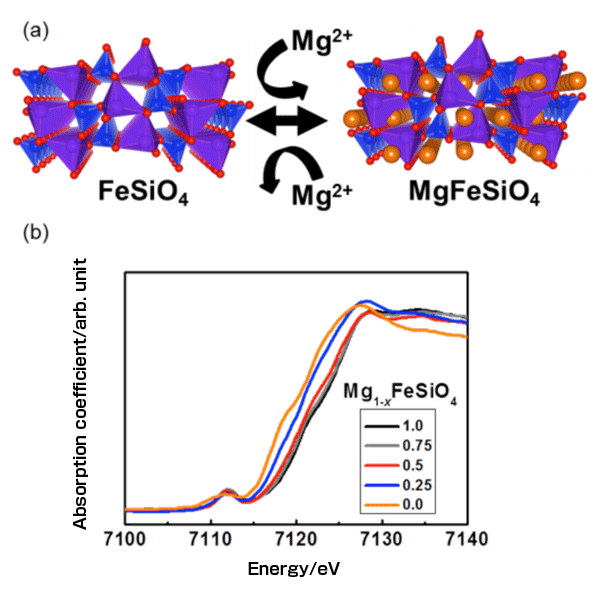
(b) X-ray absorption spectra at the Fe K-edge during Mg ion insertion. The energy shifts of the X-ray absorption near-edge structure correspond to the oxidation state of Fe.
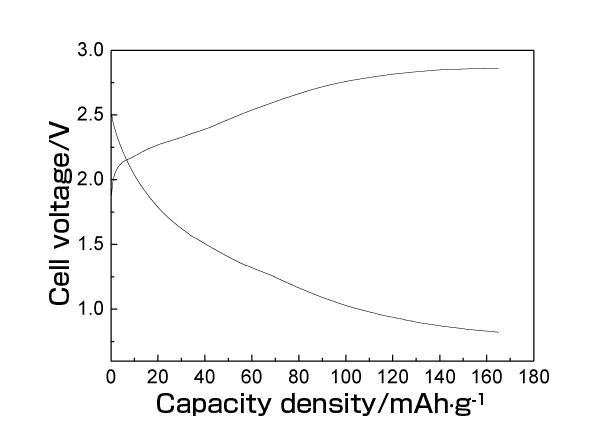
The upper right shows the region of higher energy density.
《Glossary》
※1 Rechargeable Mg batteries
Rechargeable batteries with stable Mg metal as an anode. Rechargeable Mg batteries are expected to serve as the next-generation rechargeable battery system that realizes higher energy density, greater safety, and lower cost than lithium ion batteries, which are most widely used at present. The research and development of rechargeable Mg batteries has been accelerated since 2000, but there are still many issues to resolve.
※2 Lithium ion batteries
Rechargeable batteries with high energy density used as the main power sources for mobile devices such as mobile phones and laptops. Recently, they have been adopted in hybrid cars, electric vehicles, aircraft, and large-scale storage batteries. Lithium ion batteries mainly consist of electrodes (an anode and a cathode) and an organic electrolyte solution undergoing charge-discharge reactions via the transfer of lithium ions. For the use of lithium ion batteries as power sources in vehicles, further improvement in safety, as well as an increase in size, is essential.
※3 SPring-8
A synchrotron radiation facility that provides the world’s highest-brilliance synchrotron radiation. It is owned by RIKEN and located at Harima Science Park City, Hyogo Prefecture, Japan. JASRI is responsible for the operation, management, and promotion of the use of SPring-8. When the direction of the electron beams accelerated to nearly the speed of light is changed by magnets, electromagnetic waves are emitted in the tangential direction; these waves are synchrotron radiation. SPring-8 is used to advance research activities in various fields such as materials science, earth science, life science, environmental science, and industrial science.
|
For more information, please contact: |
- Previous Article
- Direct Observation of “Hidden Order” That Has Been a Mystery in Materials Physics (Press Release)
- Current article
- Successful Development of High-Energy-Density, High-Safety, and Low-Cost Rechargeable Batteries – From lithium to magnesium metal - (Press Release)