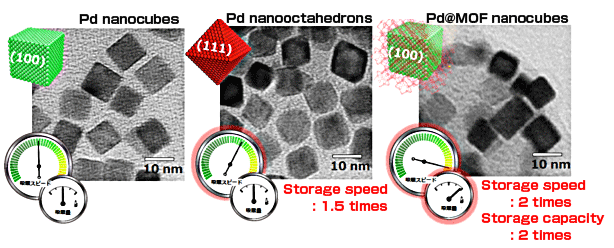
Porous Metal Complex Changes Properties of Palladium - Hydrogen storage capacity and storage/release speed in palladium are doubled - (Press Release)
- Release Date
- 14 Jul, 2014
- BL02B2 (Powder Diffraction)
July 9, 2014
Kyoto University
Japan Science and Technology Agency (JST)
|
A research group led by Hiroshi Kitagawa (professor) of the Graduate School of Science, Kyoto University, succeeded in changing the hydrogen storage speed of palladium (Pd) nanocrystals by precisely controlling the surface atomic arrangement of those nanocrystals. They also found that the Pd nanocrystals covered with a porous metal complex*1, namely, a metal-organic framework (MOF) composed of metal ions and organic ligands, have twice the hydrogen storage capacity and the hydrogen storage/release speed of bare Pd nanocrystals. In addition, they clarified that the dramatic enhancement of hydrogen storage properties was a result of the charge transfer at the interface between Pd nanocrystals and MOF. This new material is highly expected to be utilized as a hydrogen storage material, hydrogen separation membrane, and electrocatalyst for fuel cells as well as a high-efficiency hydrogenation catalyst. Pd is one of the platinum group elements with a face-centered cubic (fcc) lattice structure. It is useful in various catalysts such as hydrogenation catalysts, catalysts for purifying automotive exhaust gas (three-way catalysts), and electrocatalysts for fuel cells. At the same time, Pd can store approximately 1,000 times its own volume of hydrogen and therefore is being actively studied for practical use as a hydrogen storage metal*2 and hydrogen separation membrane*3. Alloying of Pd with dissimilar metals is currently employed to further enhance the performance of Pd. By preparing shape-controlled Pd nanocrystals, the research group of this study succeeded in changing the hydrogen storage speed of Pd by precisely controlling the atomic arrangement of the Pd nanocrystal surface. They also found that the hydrogen storage properties, such as hydrogen storage capacity and hydrogen storage/release speed, of Pd were dramatically enhanced by covering the surface of Pd nanocrystals with an MOF that consists of organic ligands and metal ions, as shown in Fig. 1. These results suggested that the properties of metal materials are dramatically enhanced by controlling the structure of the nanocrystal surface and covering the surface with an MOF. These techniques, combined with various metals and MOFs, will enable the creation of innovative materials in the future. This study was carried out as part of the “Creation of the Functional Materials on the Basis of the Inter-Element-Fusion Strategy” project (Representative: Professor Hiroshi Kitagawa, Kyoto University) within the research scope of “Creation of Innovative Functions of Intelligent Materials on the Basis of Element Strategy” of the Core Research for Evolutional Science and Technology (CREST) supported by the Japan Science and Technology Agency (JST). Their achievements were published online in the British scientific journal Nature Materials and also in the Journal of the American Chemical Society Publication: |
《Figures》
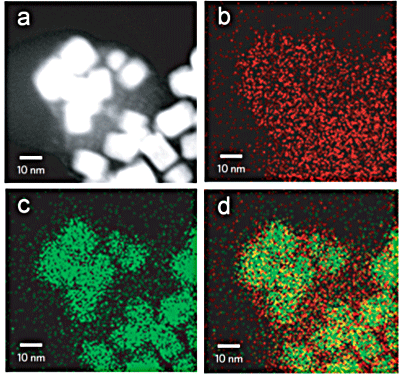
(a): HAADF-STEM image.
(b): Mapping of Cu in HKUST-1.
(c): Mapping of Pd.
(d): Superposition of elemental maps of Cu and Pd. The Pd nanocubes are covered with a nanofilm of HKUST-1.
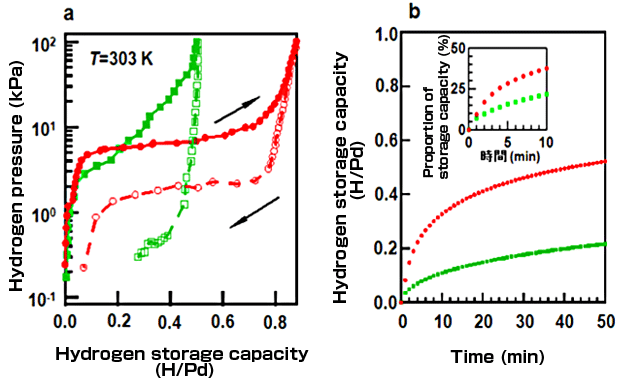
(a):Hydrogen pressure–composition isotherms. (b):Measured hydrogen storage speed.
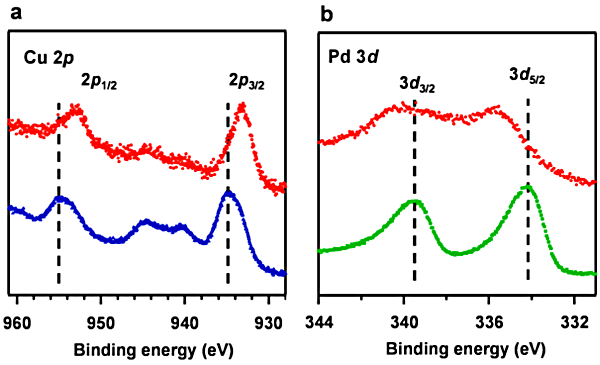
(a): Cu 2p spectra and、
(b): Pd 3d spectra. When the Pd nanocubes are covered with HKUST-1, the Cu 2p peak resulting from HKUST-1 shifts to the low-binding-energy side while the Pd 3d peak shifts to the high-binding-energy side. These results show that the hybridization induces the charge transfer from Pd to HKUST-1.
《Glossary》
*1Porous metal complex
A metal complex consisting of organic ligands and metal ions and with regular pore structures.
*2Hydrogen storage metals
Metals that can reversibly store and release hydrogen. Hydrogen molecules dissociate into hydrogen atoms on the metal surface and the atoms occupy interstitial sites in the metal lattice.
*3Hydrogen separation membranes
Membranes that separate hydrogen from hydrogen-containing mixture gas by means of a metal membrane permeable only to hydrogen. Pd and Pd alloys are widely used as hydrogen separation membranes.
*4High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM)
A type of electron microscopy in which a finely focused electron beam is scanned over a sample and the transmission electrons scattered at high angles are detected using an annular detector.
|
For more information, please contact: |
- Previous Article
- Direct Observation of “Hidden Order” That Has Been a Mystery in Materials Physics (Press Release)
- Current article
- Porous Metal Complex Changes Properties of Palladium - Hydrogen storage capacity and storage/release speed in palladium are doubled - (Press Release)