Elucidating the mechanism of recognition and evasion in viral infection of tomato -Towards the development of new anti-viral agent– (Press Release)
- Release Date
- 20 Aug, 2014
- BL38B1 (Structural Biology III)
National Institute of Agrobiological Sciences
Osaka University
Iwate Medical University
|
The structural basis in which the virus resistance protein found in wild tomato binds with the tomato mosaic virus protein to inhibit its propagation has been clarified. The virus resistance protein of tomato and the tomato mosaic virus protein each undergoes repetitive changes resulting in the host recognition of the viral molecule, viral adaptive evasion of the recognition, host counteradaptation, and viral response to counteradaptation. Such information on the structural basis of recognition and evasion could be used in developing an efficient plant anti-viral agent that can be expected to control the spread of viral diseases and an increase in the yield of affected crops. Publication: |
《Figures》
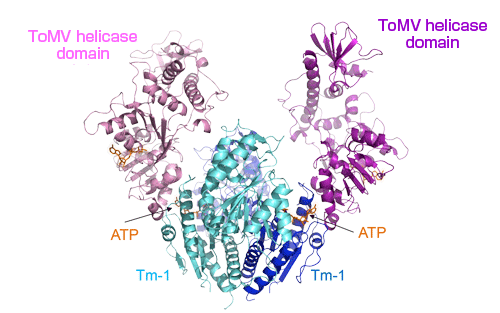
The Tm-1 protein (colored blue and cyan) forms a dimer which binds with the ToMV helicase domain (colored pink and violet). An ATP molecule exists at the contact surface of each molecule.
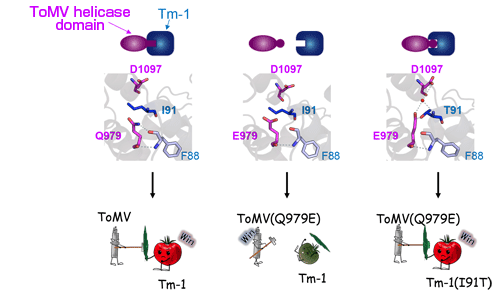
(Left) Tm-1 binds with ToMV helicase to inhibit propagation of the virus. In this case, the Tm-1 amino acid no. 91 isoleucine (I91) interacts with the ToMV-Hel amino acid no. 979 glutamine (Q979) and no. 1097 aspartic acid (D1097). (Middle) Substitution of Q979 with glutamic acid (E979) allows ToMV to evade inhibitory interaction with Tm-1. (Right) Substitution of I91 with threonine (T91) in Tm-1 restores an interaction with E979, thereby inhibiting the propagation of the virus with E979 mutation.
|
For more information, please contact: |
- Current article
- Elucidating the mechanism of recognition and evasion in viral infection of tomato -Towards the development of new anti-viral agent– (Press Release)