Successful Observation of Single-Molecule Intramolecular Motions of Ligand Gated Ion Channel in Real Time -Establishment of X-ray technology for allosteric drug design- (Press Release)
- Release Date
- 16 Sep, 2014
- BL40XU (High Flux)
University of Tokyo
Advanced Industrial Science and Technology (AIST)
Public University Corporation of the University of Hyogo
Japan Synchrotron Radiation Research Institute (JASRI)
Key points
♦ Dynamic internal motions of nicotinic acetylcholine receptor,nAChR, a key functional protein responsible for muscle movement, memory and learning, were observed at single molecule level with 100 μs time resolution for the first time in the world.
♦ It was distinguished the characteristic intramolecular motion of home-pentameric protein (AChBP)from hetro-pentameric protein(nAChR)by single molecule analysis with diffracted X-ray tracking.
♦ The developed monitoring technique for obtaining internal dynamic information on the subunits can be a fundamental monitoring technique for discovering drugs without side effects.
|
Aresearch group led by Yuji Sasaki (professor) at the Graduate School of Frontier Sciences, the University of Tokyo, successfully observed dynamic three-dimensional (3D) single-molecule internal motions of nAChR, a high-profile protein that controls muscle motion, memory, and learning ability, at a 100 μs time resolution and picometer (a length one-hundredth of the diameter of an atom) accuracy for the first time in the world. The group members include Hiroshi Sekiguchi (research scientist) at the Japan Synchrotron Radiation Research Institute, Tai Kubo (Deputy Director) at the Molecular Profiling Research Center for Drug Discovery, the National Institute of Advanced Industrial Science and Technology, Atsuo Miyazawa (professor) at the Graduate School of Life Science, University of Hyogo, and Masato Okada (professor) at the Graduate School of Frontier Sciences, the University of Tokyo. Publication: |
《Glossary》
*1 heteropentamers
Protein composed of hetero- and homo-subunits Most of proteins are molecular complex that composed of multiple subunits that are called oligomer or oligomeric proteins. Some olygomeric proteins have identical or homologous subunits, and others have completely different subunits that are responsible for totally different functions. Oligomeric protein comprising the subunit of an identical structure are homo-oligomers, whereas those comprising different types of subunit are hetero-olygomers.
*2 Diffracted X-ray tracking(Diffracted X-ray Tracking; DXT)
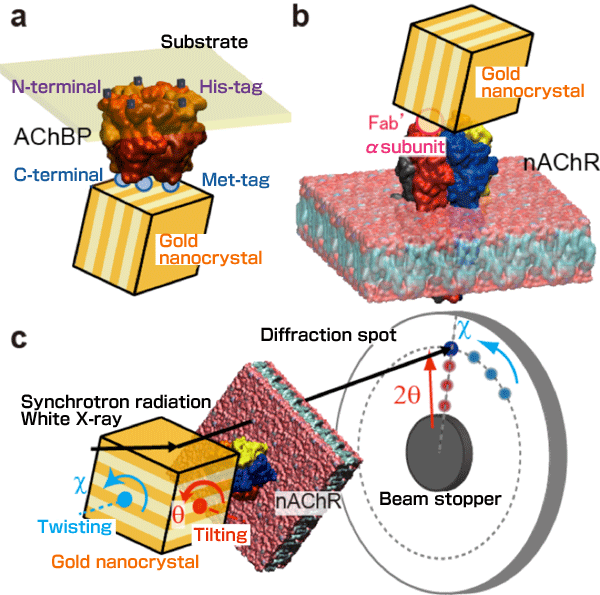
DXT is a powerful technique in biological science for detecting atomic-scale dynamic motion of the target protein at the single molecular level at several tens of microseconds time resolution. The dynamics of a single protein can be monitored through trajectory of a Laue spot from a nanocrystal which was attached to the target protein immobilized on the substrate surface Professor Yuji Sasaki designed this technique in 1997 and presented it in 2000. He has monitored the internal motions of various proteins and reported the results in many journals (e.g., Physical Review Letters, Physical Review, BBRC, Cell). The figure below shows schematics of the DXT technique applied to (a) acetylcholine-binding protein (AChBP) and (b) nAChR, and (c) a schematic of the DXT experimental setup. A movie is also provided as supplementary material to show the motions of the nAChR.
*3 Intramolecular dynamics
In general, intramolecular dynamics is a generic term for the motions of protein molecules that occur upon the expression of their functions. In particular, ion channel molecules examined in this study bind to ligands and the central region of the molecules far from the binding site is considered to change greatly. However, this motion has been predicted simply by comparing the stable structural information before and after the structural transition. An issue for conventional monitoring techniques has been the realization of real-time observation on a time axis because the intramolecular dynamics (motions) of protein molecules is closely related to their functions. In addition, accurately monitoring the motions of individual molecules requires a single-molecule monitoring technique. The DXT technique was successfully applied to single-molecule monitoring for the first time in the world.
*4 Allosteric drug discovery
Allosteric drugs have recently been attracting attention. Allosteric drugs can minutely regulate the activity of a target molecule by binding to a site other than the active center of the molecule that usually targeted by medications. These drugs might be highly promising because they cause no adverse effects. Conventional drugs attach tofatal sites of the target molecule, which causes unnatural reactions as side effects of the drugs. “Allosteric” originates from the Greek word “allostery”, which is a combination of “allos” meaning “other” and “stereos” meaning “solid”. In short, the allosteric effect is the conformational change of a molecule at a site other than the specific site on which the molecule acts. This change occurs via the high-speed cooperative transfer of intramolecular motions. However, there had been no method of rapidly and accurately monitoring the intramolecular motions. The DXT technique was the first to realize such monitoring. The allosteric effect was proposed by Jacques Monod (who was awarded the Nobel Prize in Physiology or Medicine in 1965), Jeffries Wyman, Jean-Pierre Changeux, and others. It also stands for molecular cooperativity.
|
For more information, please contact: |
- Current article
- Elucidating the mechanism of recognition and evasion in viral infection of tomato -Towards the development of new anti-viral agent– (Press Release)