Toll-Like Receptor 8 (TLR8) Recognizes Degradation Products of RNA -Uridine is essential for activation of TLR8- (Press Release)
- Release Date
- 20 Jan, 2015
- BL41XU (Structural Biology I)
- BL45XU (RIKEN Structural Biology I)
University of Tokyo
Key points
•Toll-like receptor 8 (TLR8) is a protein that activates innate immune responses to protect living organisms from viral and bacterial invasion.
•The detailed crystal structures of TLR8 in complex with single-stranded RNA (ssRNA)*1 were clarified. It was also clarified that the coordinated interaction between ssRNA and uridine, a degradation product of ssRNA, is involved in the activation of TLR8 by ssRNA.
•The achievements of this study are expected to lead to the development of anticancer drugs targeting TLR8 and therapeutic agents for autoimmune diseases.
|
A research group has succeeded for the first time in the world in clarifying the detailed crystal structures of TLR8 in complex with ssRNA. TLR8 activates the innate immune system by recognizing ssRNAs derived from pathogens. The research group was led by Toshiyuki Shimizu (professor), Hiromi Tanji (graduate student), and Umeharu Ohto (lecturer) of the Graduate School of Pharmaceutical Sciences, The University of Tokyo, and Kensuke Miyake (professor) and Takuma Shibata (assistant professor) of the Institute of Medical Science, The University of Tokyo. Publication: |
<<Figures>>
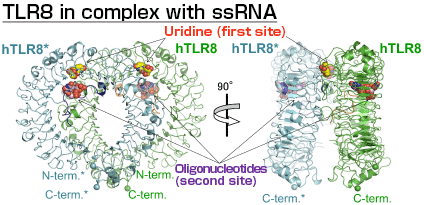
One unit of a TLR8 dimer is indicated in green and the other unit is in blue. There are two ligand-binding sites; uridine binds the first site (red) and oligonucleotides bind the second site (purple).
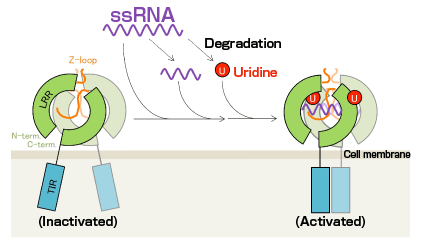
Diagram of mechanism of TLR8 activation by ssRNA. Uridine (a degradation product of ssRNA) binds to the first site, and ssRNA and oligonucleotides (degradation products of ssRNA) bind to the second site. ssRNA and uridine coordinately activate TLR8.
<<Glossary>>
[1] Single-stranded RNA (ssRNA)
ssRNA is an RNA that is not a double-stranded RNA. Viruses that have their genetic information and RNA (RNA viruses) are classified into ssRNA or double-stranded RNA.
[2] Uridine
Uridine is a constituent of RNA and has a structure in which uracil (a base) is bound to ribose (a sugar).
|
For more information, please contact: |
- Previous Article
- Electronic rubber band effect makes efficient intelligent catalysts (Press Release)
- Current article
- Toll-Like Receptor 8 (TLR8) Recognizes Degradation Products of RNA -Uridine is essential for activation of TLR8- (Press Release)