Creation of New “Materials” by Nanotechnology
New materials created one after another
We can make our lives more convenient by exploiting the properties of materials around us. From the opposite viewpoint, the properties of materials determine the convenience of our lives. If new materials are discovered in the future, our lives will greatly change. Such discovery is ongoing in the nanometer world (a nanometer is one-billionth of a meter).
As a typical example, fullerene was discovered in 1985 (Fig. 1). This newly discovered material attracted much attention at the time because its characteristic shape, comprising 60 carbon atoms, is similar to a soccer ball. In 1991, a cylindrical carbon nanotube was discovered and overturned the commonly held beliefs in nanotechnology. In January 2008, Associate Professor Ryo Kitaura and Professor Hisanori Shinohara at Nagoya University succeeded in synthesizing a nanometer-scale metal wire.
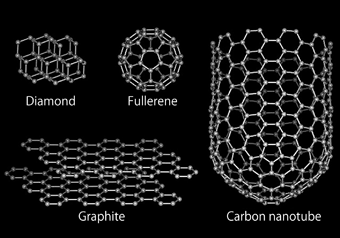
Fig. 1 Carbon allotropes.
Carbon allotropes were conventionally considered to be diamonds and graphite. Fullerene and carbon nanotubes have been added to this group.
The smallest world
“In many East Asian countries, 10-9 is called jin, which means dust. Nanotechnology is exactly that, technology at the scale of dust. The world smaller than the nanometer scale is in the interior of atoms,” says Professor Shinohara. That is, the smallest scale directly linked to us is the nanometer.
In 1959, Dr. Richard Feynman, an American Nobel-Prize-winning physicist, insisted, “When we try to investigate things on an increasingly smaller scale, we reach a limit, but this is where there is plenty of room for research.” That was the beginning of research on nanotechnology. In 2000, former US President Bill Clinton mentioned nanotechnology as an emerging technology in his State of the Union address, which made nanotechnology widely known.
The recent progress in the research on nanotechnology has mostly been due to the advancement of nanoscopic observation technology achieved since the mid 1980s. For example, the scanning tunneling microscope, which can reveal the atomic-level structure of the surface of a material from the current induced by bringing a sharp-pointed probe close to the surface, was developed in 1982. The concept of constructing SPring-8 to observe microstructures was first proposed in 1984.
Thus, nanometer-scale materials have become available, and their characteristic properties are now used in various applications. For example, fullerene is added to sports equipment to increase the strength of plastics. Fullerene is also used in cosmetics because ultraviolet-induced substances harmful to our skin are absorbed by the spherical structure of fullerene.
As another example, carbon nanotubes are expected to be highly helpful in the safe transport of explosive hydrogen gas because they can adsorb gas. In addition, expectations are cast on the application of carbon nanotubes to electronic devices because of the phenomenon that their electrical conductivity greatly changes in accordance with the rolled state of nanotubes (Fig. 2).
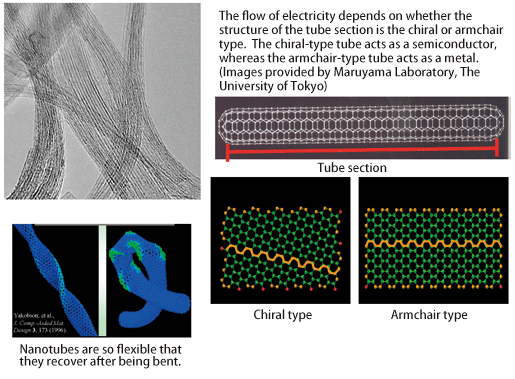
Fig. 2 Electron microscopy image of carbon nanotubes (upper left) and their features.
Metal nanowire
In research into nanotechnology of interest, Professor Shinohara and his colleagues have synthesized a metal nanowire using a carbon nanotube as a chemical reactor.
If you mix a carbon nanotube and fullerene, heat them to 500°C, and react for two days, fullerene molecules enter the nanotube (Fig. 3). This condition is similar to peas in a peapod, and because of this, the structure is called a peapod. Professor Shinohara thought that they could produce something new by combining the metal-containing fullerene (fullerene in which metal atoms are contained), which he has intensively studied, and the peapod, with the hope of discovering new materials in the nanometer world.
Professor Shinohara and his group created a peapod using fullerene molecules that contain gadolinium (Gd) atoms and heated it at a high temperature of 1200°C. This causes fullerene molecules to break and the Gd atoms are successfully ordered in the carbon nanotube. This indicates that a metal nanowire covered with a carbon nanotube can be created.
However, the wire was fragmented in the carbon nanotube because the metal filling rate is insufficient in the method using metal-containing fullerene. He innovatively placed carbon nanotubes and erbium chrolide (ErCl3), instead of fullerene, in a test tube and heated them in vacuum at 800°C for three days. He hypothesized that ErCl3 molecules were closely packed in the carbon nanotube and formed a wire by electron microscopy (Fig. 4).
To demonstrate that the wire materials are actually ErCl3, X-ray analysis was required. General laboratories, however, have no equipment that can generate a high energy to observe the inner structure of the nanotube. Professor Shinohara sent Associate Professor Kitaura and graduate students, Daisuke Ogawa and Naoki Imazu, to SPring-8. They carried out experiments using the beamline for soft X-ray spectroscopy of solids (BL25SU) in a joint research with Tetsuya Nakamura, the senior scientist at JASRI, and Tsuyoshi Saito, the team leader at the National Institute of Advanced Industrial Science and Technology. They observed X-ray absorption that is unique to Er, thus confirming that an ErCl3 wire was formed in the carbon nanotube (Fig. 5).
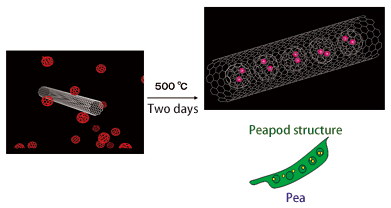
Fig. 3 Carbon nanotube takes in fullerenes. This looks like a pea in a peapod.
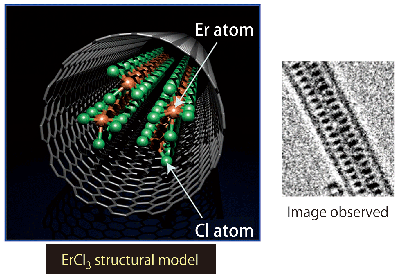
Fig. 4 ErCl3 nanowire formed in carbon nanotube.
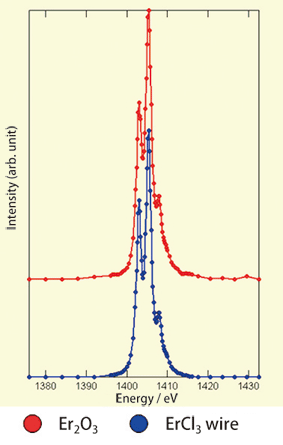
Fig. 5 Ultrahigh-sensitivity spectroscopic measurement using SPring-8 beamline for soft X-ray spectroscopy of solids (BL25SU). The absorption peak unique to Er was observed at 1409 eV.
In search of the ultimate nanowire
“Thanks to the achievement of our research in SPring-8, we have carried out subsequent experiments with confidence,” says Professor Shinohara.
Following ErCl3, the syntheses of nanowires of nickel bromide (NiBr2) and europium (Eu) were also successful. Currently, it is possible to synthesize an ultimate metal nanowire that is composed of a sequence of atoms and that cannot be any thinner.
Pure metals are easily oxidized because of their high reactivity to oxygen. Nonetheless, such a very thin nanowire can stably exist in air and solution when it is covered with a stable carbon nanotube. This is akin to an ordinary copper wire covered with plastic, and has high potential for use in ultrasmall electron devices.
“Nanowires are now attracting much attention, and our work is followed by researchers worldwide,” says Professor Shinohara. “Through our research, nanometer-scale materials have been found to have a unique stable structure. It is fun to explore the unknown features of materials attributable to their unique structure.” Nanotechnology will become more and more interesting in the future.
Column: Appeal of serendipity

Professor Shinohara is often invited to give lectures at high schools and always talks about the following theme: serendipity in nanotechnology. Serendipity is chance discovery. For example, fullerene was discovered by chance during an experiment on synthesizing carbon clusters (assembly) in cosmic space. The carbon nanotube was discovered in the by-products of fullerene synthesis by Dr. Sumio Iijima, then the chief scientist at NEC's Fundamental Research Laboratory. “Students who thought themselves strangers to great discovery and who were losing interest in science regain the sparkle in their eyes,” says Professor Shinohara. It goes without saying that tremendous effort and experience are required to take advantage of such good luck. Dr. Iijima had many years of experience with electron microscopy.
Listening to Professor Shinohara talk about his research, the interviewer felt that his tremendous effort and experience are, to him, part of a joyful process of learning things that he is curious about.
Writing by Akiko Ikeda (Sci-Tech Communications Incorporated)
This article was written following an interview with Professor Hisanori Shinohara at the Department of Chemistry, Nagoya University, Graduate School of Science.