Safe and Reliable All-Solid-State Battery To Be Achieved by Nanotechnology
Hopes for all-solid-state battery
Electric appliances are available in abundance around us. Many of them are supplied with electricity via outlets, whereas portable devices, such as mobile phones and laptop computers, use batteries.
A battery producing electricity comprises a cathode, an anode, and a liquid electrolyte*1 (Fig. 1). Recently, technologies used in manufacturing batteries, such as packaging technology, have been improved; however, there seems to be still no end to accidents involving the deformation, expansion, and ignition of batteries due to overheating. Also, many people may have encountered the leakage or freezing of liquid electrolyte in car batteries. These problems with the performance and safety of batteries will be solved when all-solid-state batteries using solid electrolytes are realized.
Professor Hiroshi Kitagawa at Kyoto University, who is also an invited professor of Kyushu University, and Rie Makiura, a specially appointed research associate at Kyushu University, have carried out research using nanometer-scale silver iodide (AgI) particles (a nanometer is a billionth of a meter) as an electrolyte, jointly with Masaki Takata, chief scientist, and Ken-ichi Kato, a scientist, both at RIKEN. They verified the possibility of realizing a stable solid electrolyte at room temperature for the first time in the world. This research achievement is highly regarded because of its potential and was published in the British scientific journal Nature Materials in May 2009.
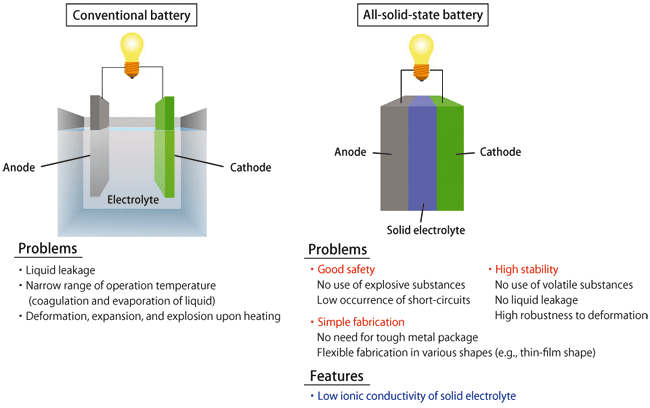
Fig.1 Structure and problems of conventional battery (left) and
expected characteristics of all-solid-state battery (right)
If a solid electrolyte is found, safer and easy-to-use batteries will become available.
Inspiration by integration of different fields
A requirement of electrolytes used in batteries is that an appropriate number of ions (electrically charged species) move at a sufficient speed. The substances previously reported to be suitable for electrolytes have all been liquids. Finding a solid alternative to an electrolytic solution involves finding a solid with ion conductivity*2 equivalent to that of an electrolytic solution.
AgI has long been known as a solid that exhibits high ionic conductivity comparable to that of liquids (a superionic conductor). However, to achieve the superionic conduction state of AgI, it is necessary to increase the temperature to 147 °C or more. This has been a barrier preventing the practical application of AgI. Many scientists consider that the physical properties of AgI cannot be changed. However, Professor Kitagawa conceived the idea of using AgI nanoparticles. His major was chemistry and he has been focusing on the fact that the physical properties of a substance change when the size of its particles is on the nanometer scale. He often attended academic meetings of physical societies owing to his interest in physics. While AgI is considered to be a potential solid electrolyte, physicists have not yet overcome the challenge of decreasing the temperature to achieve superionic conductivity. When Professor Kitagawa encountered this unsolved problem, he conceived the idea of using AgI nanoparticles. It was a natural idea for him because he knew that the melting point of gold (Au) nanoparticles is lower than that of bulk Au.
AgI nanoparticles
According to Professor Kitagawa, formation of AgI nanoparticles should not be difficult considering their practical use. By trial and error, he found a simple method of forming AgI nanoparticles involving mixing silver nitrate (AgNO3) solution, sodium iodide (NaI) solution, and a solution of the organic polymer poly-N-vinyl-2-pyrrolidone (PVP) under normal temperature and pressure, then filtering and drying the mixed solution. The resulting PVP-coated AgI nanoparticles were high-quality nanoparticles with high stability and a uniform diameter. It was also found to be possible to form various nanoparticles with different diameters by changing the forming conditions such as the proportions of AgNO3 and PVP.
Thus, Professor Kitagawa produced various nanoparticles with different diameters in the range of 10 - 40 nm, and examined the temperature at which AgI nanoparticles undergo a transition to the superionic conduction state for each diameter (Fig. 2). The result revealed that the smaller the particle diameter, the lower the phase transition temperature at which superionic conductivity is achieved. In particular, the phase transition temperature decreased to 40 °C, near room temperature, when the particle diameter was decreased to 10 nm, i.e., a feasible temperature for their practical application.
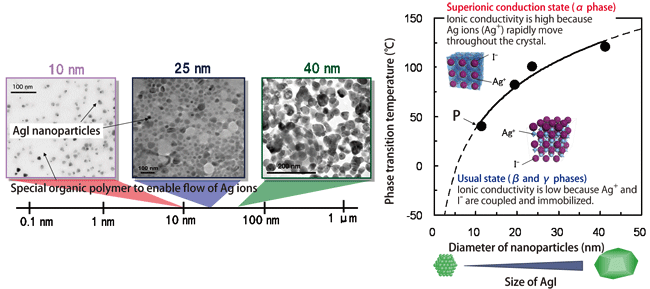
Fig.2 Developed AgI nanoparticles (photographs) and phase transition temperature at which nanoparticles with each diameter undergo a transition to the superionic conduction state (graph)
AgI nanoparticles undergo a transition from the usual state (β and γ phases) to the superionic conduction state (α phase) when the temperature is increased. The smaller the particle diameter, the lower the phase transition temperature.
Worldwide recognition of research achievements
Why can AgI nanoparticles exhibit superionic conductivity at a lower temperature? To answer this question, Professor Kitagawa analyzed the crystal structure of AgI nanoparticles using Powder Diffraction Beamline of SPring-8 (Photograph 1).
AgI is an ionic compound consisting of a positive silver ion (Ag+) and a negative iodide ion (I-). At room temperature, AgI exists in β and γ phases, both of which have poor ionic conductivity. When the temperature is increased, AgI undergoes a transition to the α phase, in which Ag ions rapidly move around I ions, similarly to in a liquid. The α phase is called the sublattice melting phase, and AgI in this phase exhibits superionic conductivity. Usually, the temperature of AgI must be increased to 147 °C for the complete transition to the α phase. However, AgI nanoparticles can achieve the α phase at a lower temperature.
The proportions of α, β, and γ phases depend on the temperature. It is speculated that the larger the percentage of the α phase, the higher the ionic conductivity of AgI; however, it is necessary to determine the proportions of α, β, and γ phases to clarify this relationship. The crystal structure of AgI differs among these three phases. The SPring-8 beamline BL02B2 is suitable for the precise analysis of crystal structure in relation to its physical properties, and enables the detailed analysis of the structure of AgI in the α, β, and γ phases, in addition to their percentages. When 10-nm-diameter AgI nanoparticles were heated to 190 °C then cooled to approximately 40 °C, as shown by point P in Fig. 2, the percentage of the α phase, which exhibits superionic conductivity, was measured to be 70.7%, and those of the β and γ phases were 18.4 and 10.9%, respectively.
The data produced by Professor Kitagawa on the correlation between ionic conductivity and crystal structure were highly evaluated in Nature Materials. Further high-quality data have been requested by world-leading scientific journals.
Photograph.1
Beamline for analysis of crystal structure by powder diffraction (BL02B2) used for structural analysis of AgI nanoparticles (photograph taken by Satoru Yoshioka)
One more step toward practical application
Professor Kitagawa has already been designing the structure of an all-solid-state battery using AgI nanoparticles (Fig. 3). In the battery, Ag atoms in an anode are ionized to emit electrons, which are used to produce electricity. Ag ions at the anode move through a solid electrolyte of AgI, then reach a cathode composed of vanadium pentoxide. This research revealed that 10-nm-diameter AgI nanoparticles exhibit ionic conductivity 105 times higher than that of conventional AgI even at 4 °C, where no α phase exists. This value is sufficient to enable the use of AgI nanoparticles in practical batteries. Analytical research to clarify the mechanism of this phenomenon is continuously ongoing at SPring-8. People are eagerly awaiting the results of this research because AgI nanoparticles with a diameter smaller than 10 nm are expected to exhibit high ionic conductivity at an even lower temperature.
The above experiments were all carried out using alternating current; however, it is necessary to investigate the use of direct current to apply AgI nanoparticles to batteries. Although it is likely to be some time before all-solid-state batteries become available, research on all-solid-state batteries, which will ensure safer batteries, is steadily ongoing towards their practical use.
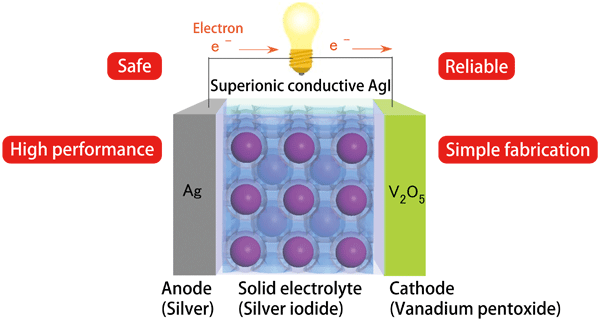
Fig.3
Schematic view of all-solid-state battery using silver iodide.
Thoroughness in everything he thinks is worthy of doing
 |
Professor Kitagawa (left) and Dr. Makiura (right) They have a high regard for each other’s work and cooperate in research. |
In his book, Professor Kitagawa introduced interpersonal skills as one of the requirements for a successful life. This is because he thinks that a sociable person is blessed with opportunities to encounter various people who will help him/her. Professor Kitagawa carries out research in cooperation with Dr. Makiura, who was his student when he was an assistant professor at Tsukuba University. After entering a private company after graduation, Dr. Makiura decided to move to Kyushu University with the aim of devoting herself to more fundamental study.
Professor Kitagawa often attends various academic meetings of societies regardless of the research field to become acquainted with a wide range of people and to obtain new knowledge and ways of thinking. He also recommends his students to attend various academic meetings and to try their hand at discussion with people outside their laboratories.
Professor Kitagawa’s enthusiasm for meeting people is not limited to his place of research. Professor Kitagawa also values conversation at bars where he drops in for a break, and says that it is good to talk with people about whom he knows nothing and who do not know him either, because they help him to overcome his feeling that he has lost touch with the sentiments of the general public during his life of research.
Finally, he told us that he drove 270,000 km, equivalent to nearly seven times around the globe, in his cherished car during seven years of his student days. Professor Kitagawa’s words seem to reveal his belief that if a job is worth doing, it is worth doing well.
Interview and original text by Akiko Ikeda (Sci-Tech Communications Incorporated)
Glossary
*1 Electrolyte
An electrolyte is a substance that dissociates into positive and negative ions. Electrolytes are usually liquid and are called electrolytic solutions.
*2 Ionic conductivity
The property that ionized species (atoms or molecules in an ionic state) can move in a solid or liquid. Ionic conductivity is expressed in siemens/cm (S/cm); ionic compounds with high ionic conductivity are suitable for batteries because their ionized species can move rapidly.
This article was written following an interview with Hiroshi Kitagawa, a professor at the Division of Chemistry, Graduate School of Science, Kyoto University, who is also an invited professor at the Department of Chemistry, Faculty of Sciences, Kyushu University.