Development of General-Purpose Plastic as Strong as Steel
Mystery of polymer strings
Plastic commodities such as plastic bags and plastic buckets are made of synthetic polymers such as polyethylene and polypropylene. At the molecular level, their structures consist of long chains of carbon atoms. These “string-like” structures of polymers give rise to a softness that can be compared to the texture of biological materials. However, while the carbon chains have strengths similar to that of the covalent bond in diamond, general-purpose plastics are not as strong. The key to solving this mystery is these strings.
Professor Masamichi Hikosaka (specially appointed professor) of the Graduate School of Integrated Arts and Sciences, Hiroshima University, has focused on the polymer strings and studied the changes in their structures and their characteristics. “When a piece of thread or yarn becomes tangled and forms a knot, you can loosen the knot not just by pulling but by moving the thread smoothly so that it slides like a snake. I considered that such “tangling” and “sliding” control the structure and function of polymers.”
The starting point of Professor Hikosaka's study of polymers was living organisms. Living organisms store genetic information in chromosome DNA and control their biological processes by retrieving, transcribing, and transmitting the information. The long string-like DNA skillfully controls the information by adequately controlling its tangling and sliding by being coiled up in helices and compactly stored in chromosomes. This mechanism has something in common with synthetic polymers.
Clarification of crystallization mechanism
In polyethylene, the tangling and sliding of strings control the change of its state from liquid to solid. When a melt (liquid in which only one substance is melted) is cooled, the tangles gradually loosen as the strings slide. Crystals grow when the molecules, still connected to each other as strings, are aligned in a lattice pattern. Since not all tangles loosen at that time, the remaining tangles are pushed out of the crystals and form “noncrystalline” masses, resulting in a state of a mixture of half crystals and half noncrystalline masses. This low degree of crystallinity had been considered the reason behind the low performance, such as the low heat resistance and the low strength, of general-purpose plastics. However, the mechanism of the crystal growth itself had not been clarified.
Professor Hikosaka examined the disarray of the strings and its effects on the properties of the strings, by X-ray diffraction and other methods. He formulated the mechanism of the growth of ideal polymer crystals and that of the actual imperfect crystals by taking into consideration the effects of tangling and sliding. This is the “sliding diffusion theory of polymer crystallization” (Fig. 1) published in 1987, providing clarification of the crystallization mechanism in polymers.
In order to verify the theory, it is necessary to observe the site of crystal growth. First, some atoms gather to form “nuclei,” namely, newborn crystals. The size of such nuclei is in the nanometer (nm: one nanometer equals one-billionth of a meter) range. While the existence of such nuclei was assumed in the 1930s, the detection of nuclei had been considered impossible. Since the 1980s, researchers all over the world have been attempting to detect nuclei using synchrotron radiation but all have failed.
Kiyoka Okada, a doctoral student (a postdoctoral fellow at present) in the laboratory of Professor Hikosaka, worked on this difficult issue. The number of nuclei in the melt was very small and the signals of the nuclei were buried in noise. An original method of breaking up crystallization additives (nucleation agents) and mixing them with the melt had been developed in that laboratory, but it had not worked. Dr. Okada made efforts to uniformly disperse the nucleation agents in the melt and repeated experiments from morning till night.
One year later, the nuclei were finally detected. Then, the nucleation process was observed for the first time in the world using the BL40B2 beamline of SPring-8 during the period from 2003 to 2007 (Fig. 2).
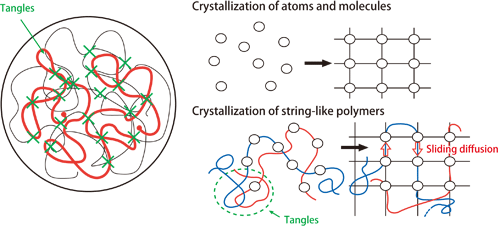
図1.Diagram of sliding diffusion theory of polymer crystallization
When atoms or molecules of low-molecular-weight substances form crystals, the individual atoms or molecules can move freely and align in a lattice pattern. On the other hand, the crystals of string-like polymers grow as the individual molecules slide and diffuse while they are still connected as strings. The tangles (indicated as red strings) are loosened, or, they are pushed out of the crystals and become solidified as noncrystalline masses. ©Masamichi Hikosaka and Kiyoka Okada, Hiroshima University
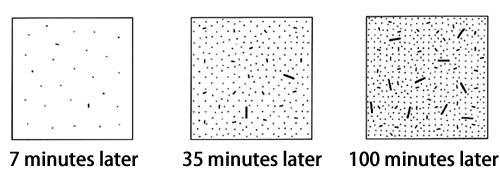
図2.Process of nucleus growth clarified from observed data obtained at SPring-8 (schematic)
At first, small nuclei begin to grow sparsely, and the number of nuclei sharply increases in 35 minutes. Larger nuclei begin to appear in 100 minutes.©Masamichi Hikosaka and Kiyoka Okada, Hiroshima University
Discovery of nano-oriented crystals (NOCs)
The next challenge for Professor Hikosaka was to create ideal crystals.
He thought it may be possible to align the molecular chains in order and to increase the degree of crystallinity by elongating the melt of polymers. However, just as water cannot be elongated, it is difficult to elongate the melt of polymers even with its high viscosity. He sandwiched and pressed the melt that was cooled to below the melting point between plates, but the elongation was insufficient.
After a process of trial and error, an idea came to him that the speed of pressing was insufficient. The speed of elongation is proportional to the speed of pressing. When the speed of pressing was increased by 200 times, the start of crystallization was observed under a polarizing microscope just 0.001 seconds after pressing. Since the crystallization in the melt started 40 minutes later when it was not pressed, it was concluded that the crystallization behavior drastically changed owing to the increase in the pressing speed.
The structure of the obtained crystals was examined using the X-ray scattering system at SPring-8 during the period from 2007 to 2010. The scattering pattern (cover images) revealed that crystals 20 to 30 nm in size were aligned (oriented) in the direction of elongation. These crystals were named “nano-oriented crystals (NOCs).” The degree of crystallinity of NOCs is 92% and the crystals are closely aligned in a row. In addition, those crystals are tightly connected to each other via a carbon chain (the covalent bond in diamond). Because the structure of NOCs looks like armor in which small iron plates are joined, it became known as the “armor model” (Fig. 3).
Professor Hikosaka's NOCs are the first case of the successful control of the tangling and sliding of synthetic polymers. It has been found that NOCs have excellent material characteristics, such as a tensile strength comparable to that of steel, toughness that allows the material to withstand bending, heat resistance that prevents the deformation of the material up to 176 °C, and glass-like transparency.
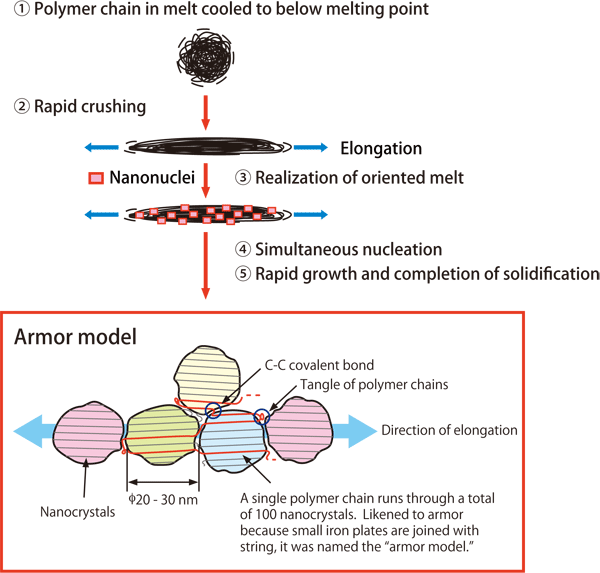
図3.Growth mechanism of NOCs
©Masamichi Hikosaka and Kiyoka Okada, Hiroshima University
Roadmap for practical applications
Plastic products began to be industrially manufactured in the 1960s. Since then, the production rapidly increased to more than 300 million tons a year (worldwide production) in 2007. While most of the plastic products are general-purpose plastics, the demand for plastics with high strength and high heat resistance, such as engineering plastics, superengineering plastics, and glass-fiber-reinforced plastics, has also been increasing. Since it is difficult to recycle these high-performance plastics because of the difficulties in melting these plastics or in isolating substances added to improve their performance, the continuous increase in the production of these high-performance plastics will lead to a serious garbage-disposal problem. In addition, they are expensive.
Because NOCs can be manufactured from the same substances as those of general-purpose plastics with an additional process of pressing, it is possible to manufacture NOCs merely by improving the existing techniques. The estimated production cost of NOCs will be from 120 to 130 yen per kilogram, which is a tenth or a hundredth of that of the conventional high-performance plastics. NOCs are expected to be applied in a wide range of areas as an alternative to the conventional high-performance plastics.
Considering NOCs to be an alternative to steel and metal materials, the strength and the tensile strength, when compared on a weight basis, are two- to fivefold higher than those of steel. In order to realize the same strength as that of a steel sheet, an NOC sheet must have twice the thickness, but its mass will be just one-quarter of that of the steel sheet. If NOC materials are used in automobile bodies, the vehicle weight can be significantly reduced.
Professor Hikosaka's laboratory is now carrying out a joint research project with a private company, aiming at the commercialization of NOCs. Through this project, they are making efforts to improve the characteristics, such as the tensile strength of NOCs, and also to apply NOC materials to commercial products such as food containers. NOC materials will be commercialized in various areas, and plastics will be further improved in the future.
Sharing passion for research and becoming partners

Professor Hikosaka always carries a piece of string. He became interested in polymer strings when he was in graduate school, and since then, he has tackled research in an unknown area from the standpoint of strings.
Dr. Okada looks back to when she first met Professor Hikosaka and says, “I just remember he said repeatedly that he had no idea with a serious face.” She decided to join his laboratory because she thought studying something unknown is real research.
The establishment of the nucleation process in crystals was a difficult task that had even been considered impossible. Dr. Okada boldly tackled this task and succeeded in observing the nuclei for the first time in the world. Professor Hikosaka highly values Dr.Okada's achievements and says, “The motivation for her continuous efforts has been the belief that studying something mysterious is real research. My colleagues always wondered how she could go on with the experiments without giving up.”
Professor Hikosaka and Dr. Okada share a passion for research, and also, the ultimate dream to understand the mechanisms of nature.
 |
|
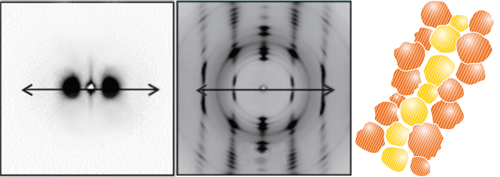
Cover images Small-angle X-ray scattering image (left) and wide-angle X-ray scattering image (right) indicating that polymer chains are aligned in the direction of elongation (arrows). The image on the rightmost is the “armor model” of NOCs. ©Masamichi Hikosaka and Kiyoka Okada, Hiroshima University |
Interview and original text: Sakiko Fukushima (Sci-Tech Communications)
This article is based on interviews with Professor Masamichi Hikosaka, a specially appointed professor, and Dr. Kiyoka Okada, a postdoctoral fellow, of the Graduate School of Integrated Arts and Sciences, Hiroshima University.