Relation between the crystal structure of the organic dye molecules and the color change induced by their crystallization
- Release Date
- 07 Apr, 2005
- BL46XU (Engineering Science Research III)
April 7, 2005
JASRI/SPring-8
|
Drs. Noritaka Kato, Kazuya Yuasa (Waseda Univ.), Mr. Takeshi Araki (Waseda Univ.), Prof. Yoshiaki Uesu (Waseda Univ.), Drs. Ichiro Hirosawa, Masugu Sato, Naoshi Ikeda (JASRI/SPring-8), and Dr. Ken-ichi Iimura (Utsunomia Univ.) have been succeeded in determining the structure of the 2-dimensional crystal of the organic dye molecules and quantitatively clarified the relationship between the crystal structure and the crystal color. The work is published in Physical Review Letters, 8 April 2005. |
Drs. Noritaka Kato, Kazuya Yuasa (Waseda Univ.), Mr. Takeshi Araki (Waseda Univ.), Prof. Yoshiaki Uesu (Waseda Univ.), Drs. Ichiro Hirosawa, Masugu Sato, Naoshi Ikeda (JASRI/SPring-8), and Dr. Ken-ichi Iimura (Utsunomia Univ.) have been succeeded in determining the structure of the 2 dimensional crystal of the organic dye molecules and quantitatively clarified the relationship between the crystal structure and the crystal color.
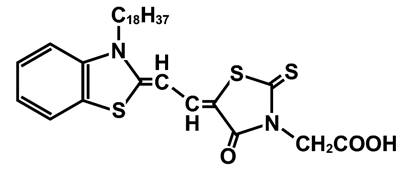
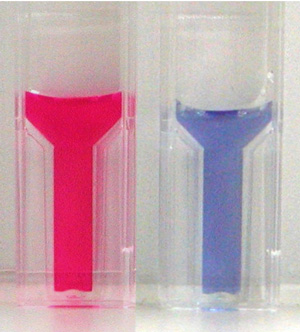
In general, the color of organic dye molecules changes by crystallization. When the visible absorption peak shifts to longer wavelength upon the crystallization, such crystals are called as J-aggregates. The J-aggregates has been used as sensitizers for silver halides in conventional photography for long time, and in recent, they attract attention as candidates for ultra fast nonlinear optical materials. Amphiphilic merocyanine (MC) dye molecules (Fig. 1) form a monomolecular-thick film (monolayer) at the air-water interface. The monolayer consists of J-aggregates (two-dimensional crystals) of MC molecules. Upon this crystallization, the color of MC shifts from red into blue (Fig. 2), and the peak shift of the visible absorption band corresponds to ΔE = - 0.36 eV. To understand this peak shift (color change), the crystal structure has to be known exactly.
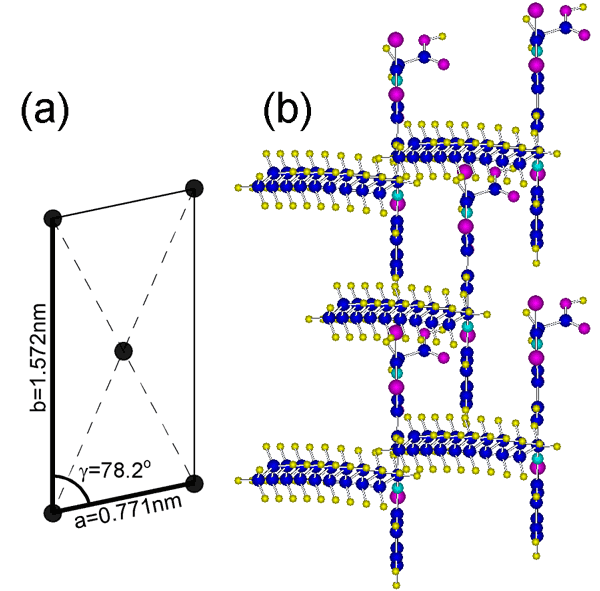
To determine the crystal structure, grazing incidence X-ray diffraction (GIXD) measurements were performed on the MC J-aggregate monolayer at the air-water interface at the R&D(2) Beamline, BL46XU, at SPring-8, and the structural analysis has been done successfully (Fig. 3). To evaluate the relationship between the crystal structure and the peak shift (ΔE) quantitatively, the intermolecular interactions were calculated on the determined crystal structure. On the calculation, simple formulas for the electric dipole interaction energy and the transition dipole moment interaction energy were derived and used. The farmer interaction is originated from the difference between the electric dipole in the ground state and the first excited state of the molecule, and this interaction was not duly taken into account so far. According to the result, it was found that the electric dipole interaction contributes significantly (about 50% of the ΔE), indicating that the electric dipole interaction also plays an important role in the peak shift.
Due to their advantages of cost performance and flexibility, the optical devices using crystals or aggregates of organic dye molecules, e.g., dye-sensitized solar cells, electro-luminescence devices, CD-R, DVD-R etc., are attracting much attention. Wavelengths of the absorption peaks of the devices are important, because they give the wavelengths, where the devices work. For examples, the maximum response of the optical sensitization or the nonlinear optical effect can be obtained at the wavelength of the absorption peak, and the luminescence appears at the wavelengths close to the absorption peak. By using the derived formulas that can be applied to any kind of organic dye molecules, the molecular arrangement in the crystal can be estimated from the peak shift (ΔE), and vice versa. Thus the formulas obtained by the present result might be useful for developing and designing the optical devices based on the organic dye molecules.
This work was supported by a Grant for The 21st Century COE Program (Physics of Self-Organization Systems) at Waseda University from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
|
||
|
||
|
|
For further information, please contact: Dr. Noritaka Kato (Waseda University) Dr. Ichiro Hirosawa (JASRI/SPring-8) Public Relations Office, JASRI |
- Previous Article
- Top-up Operation Starts at SPring-8 Storage Ring
- Current article
- Relation between the crystal structure of the organic dye molecules and the color change induced by their crystallization