Determination of the Crystal Structure of the Solid Oxygen ε-Phase Unsolved for a Quarter Century - Discovery of a red oxygen O8 cluster - (Press Release)
- Release Date
- 06 Sep, 2006
- BL10XU (High Pressure Research)
September 6, 2006
National Institute of Advanced Industrial Science and Technology (AIST)
University of Hyogo
Japan Synchrotron Radiation Research Institute (JASRI)
Key Points
• We have succeeded in the determination of the structure of the red oxygen ε-phase formed under pressures above 10 GPa.
• We have discovered an O8 cluster, which is a new conformation of oxygen following ozone.
|
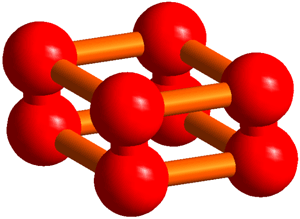
Synopsis The solid oxygen ε-phase formed under pressures from 10 to 96 GPa (1 gigaPascal = 109 Pascal) was discovered in 1979, but until now the structure has been unclear, despite many experimental and theoretical investigations. AIST, in collaboration with the University of Hyogo and JASRI, has succeeded in the determination of the structure by powder X-ray diffraction experiments at SPring-8 synchrotron radiation facility and structure analyses. In the structure, an O8 cluster consisting of four O2 molecules has been discovered (Figure 1), which nobody had theoretically predicted. We think that this structure may greatly influence the structural investigation of elements as a new conformation of diatomic molecules following ozone. Article: |
Background to Research Work
Investigation of the molecular dissociation and metallization of molecular solids has been a long-term problem in the fields of solid state physics and earth and planetary sciences. The investigation of the pressure-induced metallization and molecular dissociation process of the simplest diatomic molecules, such as H2, N2, O2, F2, Cl2, Br2, and I2, has for a long time attracted much attention.
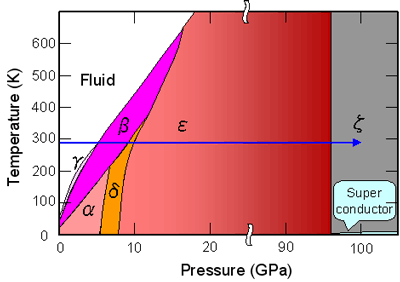
It has been known that oxygen is solidified to take a state called the β-phase at room temperature by applying pressure, and with further increasing pressure, the β-phase undergoes phase transitions to the δ-phase at 9 GPa and the ε-phase at 10 GPa; and, due to the increase in molecular interactions, the pink color of the β-phase changes into orange (δ-phase) and red (ε-phase), and the red color of the ε-phase further changes to black with increasing pressure (Figure 2).
The University of Hyogo has recently found that a ζ-phase appears at 96 GPa when ε-phase oxygen is further pressured. The ζ-phase with metallic luster has been known to exhibit superconductivity at low temperature. Oxygen molecules are one of the very few molecules having magnetic moments, and have attracted attention from the viewpoint of the relationship between the molecular magnetization and crystal structures, electronic structures, and superconductivity. However, the crystal structure of the ε-phase has until now been unsolved in spite of many experimental and theoretical studies.
History of Research Work
Until now, as possible models for the ε-phase structure, the O4 model of a pair of O2 molecules (estimated from optical measurements) and the chain model, in which the O2 molecules are one-dimensionally connected (theoretical prediction) have been proposed. However, the diffraction patterns calculated from these models are not consistent with the diffraction patterns obtained from experiments, and thus these models have been in doubt.
By effectively combining their own techniques, the University of Hyogo, SPring-8/JASRI, and AIST have attempted powder X-ray diffraction experiments and structural analyses for the ε-phase in which neither Japanese nor foreign institutes have succeeded. The University of Hyogo has a high-pressure experimental technique for solidifying gaseous oxygen by confining it in a high-pressure device called a "diamond-anvil cell." However, this technique can prepare only very small samples (60 µm in diameter and 30 µm in thickness), and, in addition, as oxygen is a light element, X-ray diffraction intensities are weak, making it difficult to obtain high-quality diffraction patterns. However, using high-brilliance synchrotron radiation from the High Pressure Research Beamline BL10XU of SPring-8, powder X-ray diffraction patterns with sufficient intensities and resolution can be obtained even from very small samples. In addition, using an analysis technique of AIST for determining crystal structures from powder X-ray diffraction patterns, we thought that the longstanding problem could be solved.
Details of Research Work
The oxygen ε-phase was prepared as follows. First, oxygen gas and the diamond-anvil cell were cooled with liquid nitrogen, and thus the oxygen was liquidized. The liquid nitrogen was enclosed in the sample chamber of the diamond anvil cell, and pressured to solidify it. The solidified oxygen can maintain the solid state under continued applied pressure even at room temperature. Then, X-ray diffraction patterns of the solidified oxygen powders were obtained using the High Pressure Research Beamline BL10XU of SPring-8.
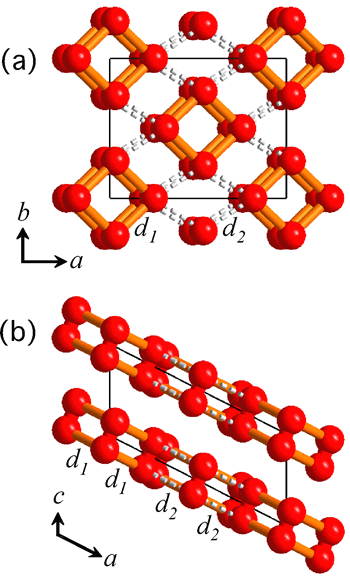
The procedure of analyses for determining the structure from the powder diffraction patterns was as follows. First, we assumed that the crystal structure of the oxygen ε-phase belongs to the lowest symmetry space group, P1, and constructed an initial model using the simulated annealing method. Secondly, considering the features of the structure, the symmetry was gradually enhanced to finally reach a model of the structure with space group C2/m. Lastly, after making the structure more precise using the Rietveld analysis, the structure obtained showed an O8 cluster in which four oxygen molecules take a box-like arrangement (Figure 3).
Thus, we have found that the structure of the ε-phase is based on an O8 cluster, but is not consistent with the O4 model estimated from optical measurements or with the chain model predicted theoretically. Further, we have confirmed that this structure is formed up to 96 GPa. The box-like cluster is a unique conformation which has first been discovered for oxygen, and has never been reported experimentally or theoretically for any other diatomic molecules.
Future Prospects
By the collaboration of three institutions, the structural analyses of the oxygen ε-phase have succeeded. Hereafter, based on the structure of the ε-phase, we plan to determine the structure of the oxygen ζ-phase formed under pressures above 96 GPa, which exhibits metallization and superconductivity. If successful, this will provide significant information to clarify the mechanisms of metallization and superconductivity of oxygen. Also, by discovering a new diatomic-molecule conformation in this work, we expect that the investigation of structural analyses for hydrogen and other elements will be accelerated.
The formation mechanism of the O8 cluster found in the work is not clear yet, and we think that the charge transfer between oxygen molecules or the magnetic moment of oxygen molecules has a significant role in the formation. Thus, we plan to further clarify the mechanism.
Figures:
The structure of the O8 cluster discovered in the solid oxygen ε-phase.
Pressure-Temperature phase diagram of oxygen.
The crystal structure of oxygen ε-phase formed at 11 GPa.
For more information, please contact:
Dr. Hiroshi FUJIHISA,
National Institute of Advanced Industrial Science and Technology (AIST),
Phone: +81-(0)29-862-6216,
Fax: +81-(0)29-862-6212,
E-mail: presec@m.aist.go.jp
- Previous Article
- Snapshot Measurement of "Color" of XFEL - Important step for developing XFEL science - (Press Release)
- Current article
- Determination of the Crystal Structure of the Solid Oxygen ε-Phase Unsolved for a Quarter Century - Discovery of a red oxygen O8 cluster - (Press Release)