Structural Determination of Environmentally Adaptive Sensor Protein in Microorganisms - Clarification of response system that accommodates to environment upon changes in light, heat, oxygen, and stress conditions - (Press Release)
- Release Date
- 14 Oct, 2009
- BL44B2 (RIKEN Materials Science)
- BL45XU (RIKEN Structural Biology I)
RIKEN
Key research achievements
• The response system switches on/off by contact/noncontact between the sensor domain and the phosphorylation catalyst domain.
• It is revealed how the response system converts environmental changes into biological signals related to phosphorylation.
• Possible application to the development of new antimicrobials causing no side effects to humans.
|
RIKEN (Ryoji Noyori, President) has determined the structure of an environmentally adaptive sensor protein in microorganisms for the first time in the world. This was achieved by Yoshitsugu Shiro, Chief Scientist, Seiji Yamada, a postdoctoral fellow for basic science, and Hiroshi Sugimoto, a senior research scientist at the Biometal Science Laboratory, RIKEN SPring-8 Center. Microorganisms can sense rapid changes in their environment, including changes in light, heat, oxygen, stress, and nutriant conditions, and survive by adapting to them. They sense such environmental changes via a sensing system called the two-component signaling system. This system comprises two types of protein, histidine kinase and a response regulator, and converts environmental changes into biological signals of phosphorylation. Histidine kinase is a protein comprising a chain of several domains, the shape of each of which has already been clarified. However, the entire structure of the protein has remained unclear, and the crucial problem of how environmental changes detected by the protein are converted into phosphorylation signals is still unsolved. The research group succeeded in determining the structure of the protein that binds the response regulator to histidine kinase of microorganisms by X-ray crystal structural analysis using the RIKEN Materials Science Beamline (BL44B2) and RIKEN Structural Biology I Beamline (BL45XU) at SPring-8. From this binding structure, the scientists clarified for the first time in the world that the conversion of information related to environmental changes into phosphorylation signals is respectively switched on and off in accordance with the contact or noncontact between the sensor domains and the catalyst domains: the former is a constituent of histidine kinase and senses environmental changes, and the latter promotes phosphorylation. The two-component signaling system does not exist in the human body but plays an important role in the life of germs and in the action of the plant hormone ethylene. Therefore, we hope that the findings obtained in this structural analysis will bring about breakthroughs beneficial to humankind, such as the creation of new antimicrobials and plant growth regulators. These research achievements were published in the American scientific journal, Structure, on 14 October 2009. Publication: |
<Figure>
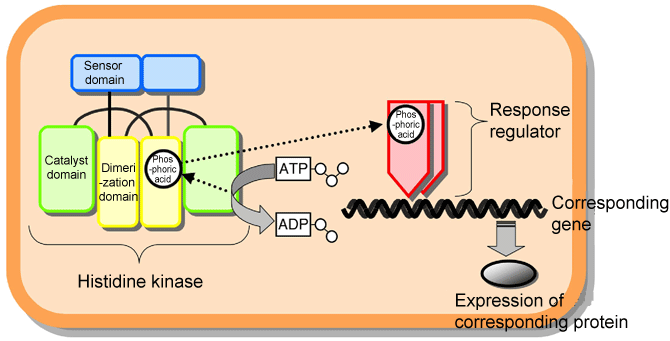 Fig. 1 Schematic of two-component signaling system used by microorganisms to adapt to the environment.
Fig. 1 Schematic of two-component signaling system used by microorganisms to adapt to the environment.
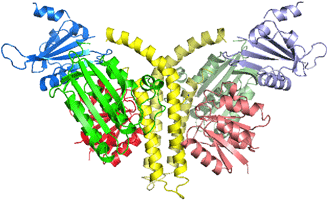 |
|||||||
|
(A) |
(B) |
||||||
|
|||||||
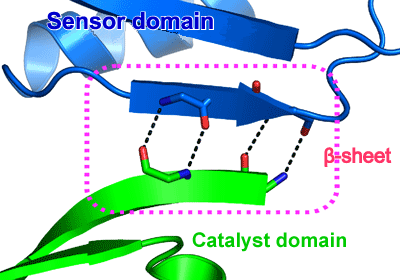 Fig. 3 β-sheet formed between sensor and catalyst domains.
Fig. 3 β-sheet formed between sensor and catalyst domains.A sensor domain (blue) is bound to a catalyst domain (green) by hydrogen bonding (dotted line), forming a β-sheet between the domains (dotted-line box).
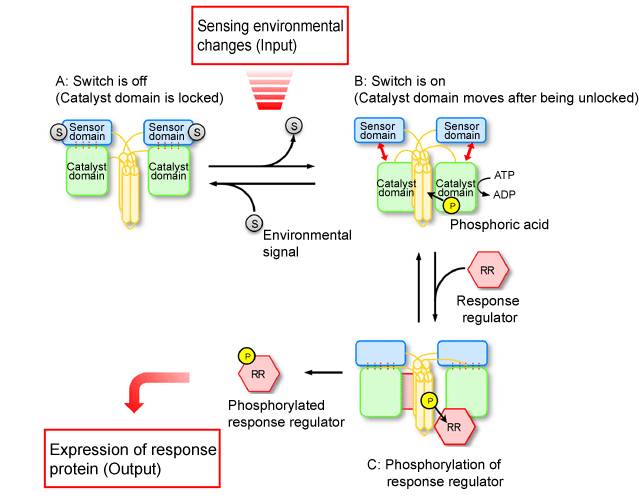 Fig. 4 Schematic of mechanism behind converting environmental changes sensed by histidine kinase into phosphorylation signals.
Fig. 4 Schematic of mechanism behind converting environmental changes sensed by histidine kinase into phosphorylation signals.
|
For more information, please contact: |
- Current article
- Structural Determination of Environmentally Adaptive Sensor Protein in Microorganisms - Clarification of response system that accommodates to environment upon changes in light, heat, oxygen, and stress conditions - (Press Release)
 .
.