New Avenues for Function Control and Application Development of Fullerenes (Press Release)
- Release Date
- 21 Jun, 2010
- BL02B1 (Single Crystal Structure Analysis)
– First-Ever Success in Large-Scale Synthesis and Single-Crystal Structure Determination of Endohedral C60 Fullerene Containing Lithium Ion -
Nagoya University
Tohoku University
Ideal Star Inc.
Japan Synchrotron Radiation Research Institute
RIKEN
|
A research group consisting of Professor Hiroshi Sawa, Associate Professor Eiji Nishibori, and Assistant Professor Shinobu Aoyagi of Nagoya University (Michinari Hamaguchi, President), succeeded in the single-crystal structure determination of a spherical molecule of endohedral C60 fullerene containing lithium ion (Li@C60),*1 which was synthesized in a large quantity (several million times larger than the conventional quantity) and refined (highly purified) by a newly developed method using the Single-Crystal Structure Analysis Beamline (BL02B1) at SPring-8 for the first time in the world. This was achieved through a joint research with groups led by Professor Hisanori Shinohara of Nagoya University and Professor Hiromi Tobita of Tohoku University (Akihisa Inoue, President), and scientists of Ideal Star Inc. (Yasuhiko Kasama, Representative Director), Japan Synchrotron Radiation Research Institute (JASRI; Tetsuhisa Shirakawa, President) and RIKEN (Ryoji Noyori, President). These achievements will accelerate the industrial utilization and application of various endohedral metallofullerenes such as Li@C60. This study showed the possibility of the stable supply and industrial application of the high-purity sample of endohedral metallofullerene using C60 fullerene as a material, which is synthesized in a large quantity. This is a pioneering report on the research on C60-based metallofullerenes, which will be widely discussed in the future, opening new avenues for the function control and application development of fullerenes. Expectation for Li@60 Quantity synthesis, structure determination and stable supply To demonstrate that the C60 fullerene molecule in the synthesized crystal contains a lithium ion, the research group of Professor Sawa at Nagoya University conducted a high-resolution single-crystal X-ray diffraction*4 experiment using a large cylindrical imaging plate (IP) camera*3 of the BL02B1 Beamline at SPring-8. They demonstrated that Li@C60 contains a lithium ion, and determined the molecular structure of Li@C60. Since lithium is an extremely light element of atomic number 3 and it is easily ionized and electrochemically activated, it is used in various fields of industry such as in ion batteries. However, it is generally difficult to examine the precise spatial state of this element due to its lightness. It is possible only by X-ray diffraction measurement using the high-brilliance X-rays at SPring-8. As a result of a detailed analysis called electron density analysis, a lithium ion incorporated in the C60 fullerene was observed, verifying the success in the isolated synthesis of Li@C60. Moreover, the lithium ion in Li@C60 was located 0.13 nm off the center, showing that Li@C60 is completely different from the inert gas molecules such as H2 and Ar. The two-dimensional arrangement of the electrically polarized Li@C60 molecules in the crystal strongly suggested the possibility of the application of Li@C60 as a single-molecule switch and a ferroelectric thin film, which has been expected on the basis of many theoretical predictions. Expectations for industrial application The use of C60 fullerene in contrast media and physiologically active materials has been conventionally attempted in the field of medical care. In addition to that field, there is a possibility that Li@C60, as a ferroelectric molecule generated by the incorporation of alkali metals, will be used in ultrahigh-density memories of more than 500 Tbit/in2 with a molecule of 1 nm diameter as one cell. The research on the application of Li@C60, the structure and electronic property of which were clarified in this study, will be further accelerated in a wide range of fields opened up by nanotechnology. These research achievements were published in the online version of the British scientific journal Nature Chemistry on 20 June 2010. Publication: |
<Figure>
](/en/news_publications/press_release/2010/100621_fig/fig1.png)
Purple: lithium ion; green: C60 fullerene; orange: SbCl6. Li@C60 molecules and SbCl6 molecules are two-dimensionally arranged in a pair.
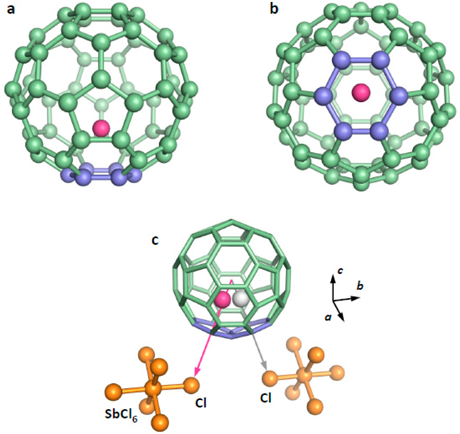
(a) and (b) are the molecular structures from different viewpoints.
The lithium ion indicated in purple is located near the six-membered ring 0.13 nm off the center of the C60 fullerene molecule indicated in green.
(c) Position of Li@C60 and two SbCl6 (orange) coordinated in vicinity. A lithium ion occupies one of the two positions near the Cl atoms adjacent to the C60 fullerene with equal probability.
<Glossary>
*1 Endohedral C60 fullerene containing lithium ion (Li@C60)
Soccer-ball-shaped spherical C60 fullerene molecule consisting of 60 carbon (C) atoms containing a lithium (Li) atom in its molecular cage denoted as Li@C60. While it has been synthesized since the 1990s, its molecular structure has not yet been clarified.
*2 Plasma shower method
Method to obtain Li@C60 by reacting lithium ion plasma generated in vacuum with C60 fullerene on negatively biased substrate. Ideal Star Inc. originally developed this method on the basis of the fundamental research by Professor Rikizo Hatakeyama and his colleagues at Tohoku University.
*3 Large cylindrical imaging plate (IP) camera of BL02B1 Beamline at SPring-8
X-ray diffraction apparatus for precise single-crystal structure analysis that was installed in the Single-Crystal Structure Analysis Beamline (BL02B1) at SPring-8 in March 2008. The X-ray detector is a large cylindrical IP with an automatic reading function. A large amount of X-ray diffraction data of a wide angular range can be collected with high accuracy and high efficiency.
*4 Single-crystal X-ray diffraction
X-ray diffraction is a method of determining the atomic arrangement in a crystal (crystal structure) and the electron distribution (electron density distribution) on the basis of the X-ray scattering pattern obtained by irradiating X-rays on crystals (X-ray diffraction pattern). When a single crystal is used as the sample, it is called single-crystal X-ray diffraction. Compared with powder X-ray diffraction, in which powder is used as the sample, the difficulty in the preparation of samples and the long period of time required for the measurement are the disadvantages. On the other hand, the small overlap of diffraction peaks and the high diffraction intensity are the advantages.
|
For more information, please contact: Prof. Hiromi TOBITA (Tohoku University) idealStar inc. Dr. Kunihisa SUGIMOTO (JASRI) |
- Current article
- New Avenues for Function Control and Application Development of Fullerenes (Press Release)