Development of Material Capable of Trapping and Decomposing Toxic Gases on Demand by Light Irradiation Japan Science and Technology Agency (Press Release)
- Release Date
- 24 Jul, 2010
- BL02B1 (Single Crystal Structure Analysis)
Kyoto University
Japan Synchrotron Radiation Research Institute
RIKEN
|
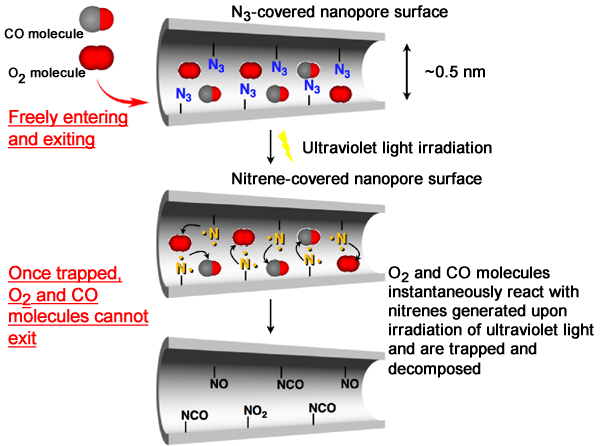
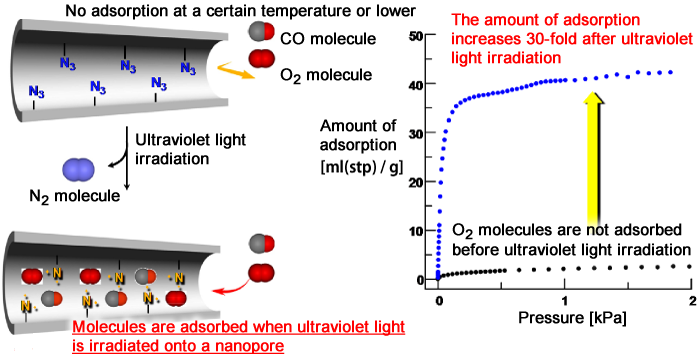
A group of scientists participating in the Basic Research Programs supported by Japan Science and Technology Agency (JST) have developed a porous material*1 that can trap and decompose molecules of toxic gases upon light irradiation (Fig. 1). The leading members of the group are Professor Susumu Kitagawa, Deputy Director of the Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University; Ryotaro Matsuda, Leader, and Hiroshi Sato, a research scientist, of the Kitagawa Integrated Pores research group of the Exploratory Research for Advanced Technology (ERATO) program supported by JST. The developed porous material consists of zinc ions and organic ligands (azidoisophthalic acid (AIP) and bipyridine). Upon the irradiation of ultraviolet light, the porous material can produce a large number of highly reactive chemical species (nitrenes*2) in its internal nanopores. It was experimentally found that the nitrenes on the surface of the nanopores effectively trapped and decomposed oxygen (O2) and carbon monoxide (CO) molecules in gases when ultraviolet light was irradiated onto the nanopores. In addition, the scientists analyzed the structural changes of the surface of the nanopores due to ultraviolet light irradiation in detail using SPring-8 synchrotron radiation. They succeeded in directly observing that nitrenes, which are difficult to observe because of their high reactivity and instability, had an orderly arrangement on the surface of the nanopores. The adsorption of molecules onto conventional porous materials is controlled by changing the temperature and pressure, whereas adsorption onto this developed porous material can be controlled by irradiating ultraviolet light. The application of this groundbreaking achievement will enable the effective separation and removal of various gases, such as O2, CO, nitrogen oxides (NOx), and sulfur oxides (SOx) in the atmosphere. It is also expected to lead to the development of materials that can contribute to the enhancement of human health and improve the global environment. This research was carried out as a collaboration between Kyoto University, Japan Synchrotron Radiation Research Institute (JASRI), and RIKEN. The results were published in the online version of the British scientific journal Nature Materials on 23 July 2010. Publication: |
<Figure>
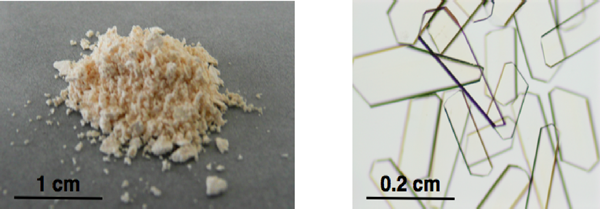
Fig. 1 Crystal powder (left) and single crystals (right) of porous coordination polymer successfully developed in this research
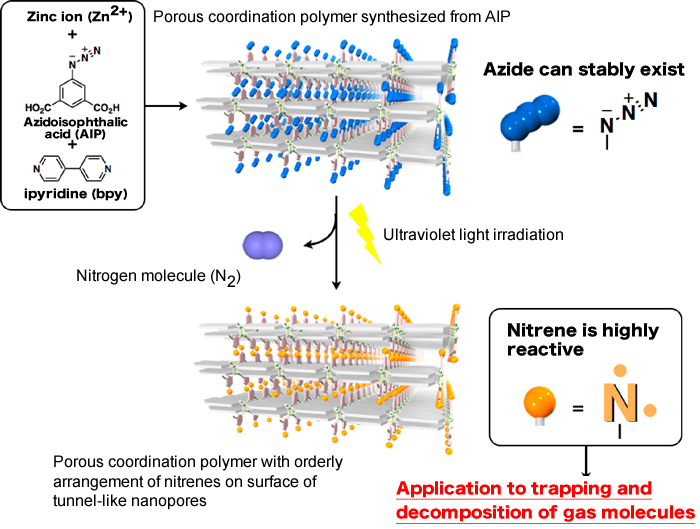
Fig. 2 Introduction of azide (N3) on surface of nanopores in porous coordination polymer to highly reactive nitrene by ultraviolet light irradiation
<Glossary>
*1 Porous material
Porous materials have a large number of pores and are used as adsorbents and catalysts. They are widely used to selectively separate and react with gases and water.
*2 Nitrene
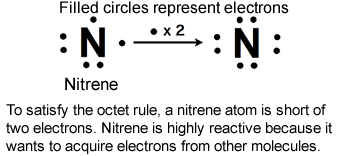 A nitrene is a chemical species containing a nitrogen (N) atom with six valence electrons. Because of the electron deficiency of the N atom, nitrenes are highly chemically reactive and are used as an intermediate in various organic chemical reactions.
A nitrene is a chemical species containing a nitrogen (N) atom with six valence electrons. Because of the electron deficiency of the N atom, nitrenes are highly chemically reactive and are used as an intermediate in various organic chemical reactions.
For more information, please contact: Prof. Susumu Kitagawa (Kyoto University) |
- Current article
- Development of Material Capable of Trapping and Decomposing Toxic Gases on Demand by Light Irradiation Japan Science and Technology Agency (Press Release)