Revealing the Mechanism of Action of New Drugs Targeting Intercellular Signaling Proteins - Expected to be applied for prevention of thrombus and antitumor effect (Press Release)
- Release Date
- 13 Jul, 2010
- BL41XU (Structural Biology I)
Nara Institute of Science and Technology (NAIST)
|
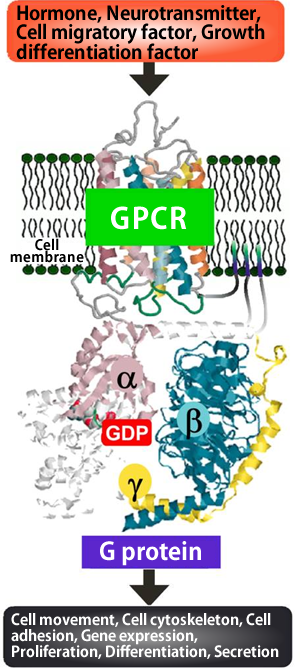
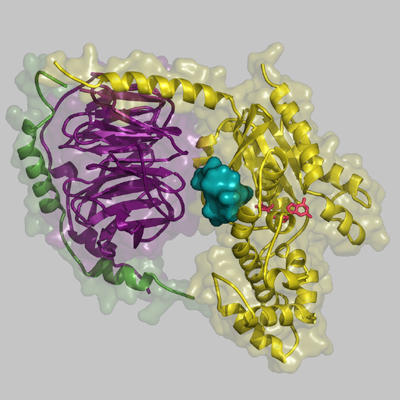
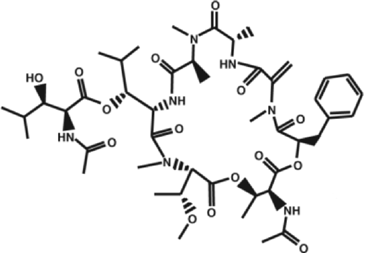
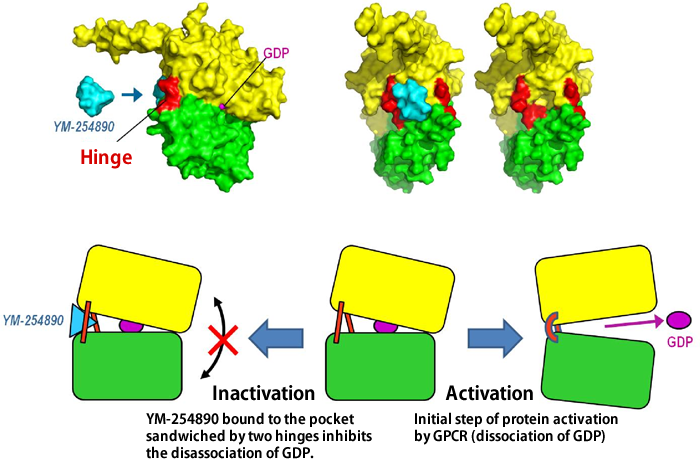
When hormones and neurotransmitters act on cells of animals, various responses may occur, such as constriction of blood vessels, nerve excitation, and blood coagulation due to the aggregation of platelets. In these responses, the guanosine triphosphate (GTP)-binding proteins (G proteins*1) in the cell membrane play a very important role of transmitting extracellular signals into the cell. A research group led by Professor Hiroshi Ito, Graduate School of Biological Sciences of Nara Institute of Science and Technology (NAIST; Akira Isogai, President), clarified for the first time in the world the steric structure and mechanism of action of low-molecular-weight compounds that block signaling by binding with G proteins. The achievements of this research may lead to a breakthrough in the development of new drugs for diseases caused by the oversecretion of hormones and neurotransmitters and malignant tumors. Moreover, they may contribute to the clarification of the mechanism underlying the activation of G proteins. The results of this research were published on 16 July 2010 in the online version of Proceedings of the National Academy of Sciences of the United States of America (PNAS). G proteins transmit extracellular signals related to hormones, neurotransmitters, cell growth factors, light, smell, and tastant into a cell. When the G-protein-coupled receptor (GPCR)*2 on the surface of the cell membrane receives such signals, the G proteins bound to the GPCR are activated to transmit the signals into the cell (Fig. 1). Humans have approximately 1,000 types of GPCR. If GPCRs are blocked, the cells do not react to signals even at excessive amounts; on the other hand, compensation for an insufficient amount of signals may lead to a treatment for diseases. Therefore, about one-half of the drugs available today target GPCRs. Also, as the activities of G proteins are regulated by GPCRs, it is possible that G proteins themselves can serve as the target molecules of new drugs. Thus far, however, the steric structures of low-molecular-weight compounds that selectively block specific G proteins and the binding sites of such compounds have not been clarified, hampering the development of new drugs. Professor Ito and his colleagues, in cooperation with Professor Toshio Hakoshima and Assistant Professor Ken Kitano at the Graduate School of Information Science of NAIST and scientists at Astellas Pharma Inc., clarified for the first time in the world the steric structures of a low-molecular-weight compound that inhibits extracellular signaling by binding with G proteins, and the complex formed by the binding of the two. (Fig. 2). For the analysis of the steric structure, synchrotron radiation X-rays of BL41XU Structural Biology I at SPring-8 were used. The achievements of this research are expected to lead to a breakthrough in the development of new drugs, as well as provide valuable information that may clarify the mechanism of activation of G proteins. For these reasons, their paper was published edited by Professor Alfred G. Gilman (The University of Texas), who received the Nobel Prize in Physiology or Medicine in 1994 for his discovery of G-proteins and the role of these proteins in signal transduction in cells. Publication: |
<Figure>
<Glossary>
*1 G proteins
G proteins are guanosine triphosphate (GTP)-binding proteins. G proteins are made up of α, β, and γ subunits. Guanosine disphosphate (GDP) or guanosine triphosphate (GTP) binds to the α subunit. By replacing GDP, which binds to the α subunit, with GTP, the G protein is activated and transmits signals into the cell. G protein functions as an enzyme that degrades GTP into GDP. The cholera toxin and pertussis toxin attach to different G proteins through a chemical structure called the ADP ribosyl group, which inhibits the functions of G proteins leading to the development of diseases.
*2 G-protein-coupled receptor (GPCR)
The G-protein-coupled receptor (GPCR) traps signals related to not only light and smell but also hormones and neurotransmitters at the surface of the cell membrane and transmits these extracellular signals to the intercellular G proteins. GPCR has a unique common structure with which GPCR can pass through the cell membrane seven times. Humans have approximately 1,000 types of GPCR. Forty to fifty percent of the drugs used currently in clinical practice target GPCRs. Many drugs, such as antihistamines, which are significantly effective for gastric ulcer, and drugs for blood pressure control, irregular heartbeat, angina, glaucoma, and Parkinson’s disease, bind to GPCR to exert their therapeutic effects.
For more information, please contact: Prof. Takao SHIMIZU (The University of Tokyo) Prof. Hiroshi ITOH (Nara Institute of Science and Technology) |
- Current article
- Revealing the Mechanism of Action of New Drugs Targeting Intercellular Signaling Proteins - Expected to be applied for prevention of thrombus and antitumor effect (Press Release)