Clarification of How Cytochrome c, an Important Protein Involved in Respiration, Loses Its Function by Joining in Chains, Half a Century after the Discovery of Its polymerization (Press Release)
- Release Date
- 07 Jul, 2010
- BL26B2 (RIKEN Structural Genomics II)
- Expected to determine the causes of diseases induced by mutation in protein structure -
Nara Institute of Science and Technology
University of Hyogo
|
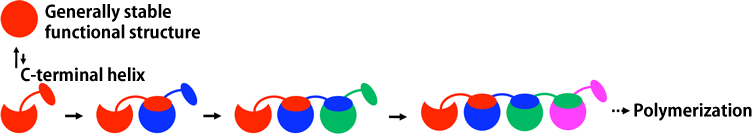
A joint research team consisting of six different groups clarified, for the first time, how cytochrome c (cyt c), a protein indispensable for respiration in living organisms, loses its function by changing its steric molecular structure and forming a polymer in which many multiple monomers are joined in chains (polymerization*1) (Figs. 1 and 2). The joint research team consists of scientists from the following: Laboratory for Supramolecular Science (Shun Hirota, Professor) and Laboratory of Bioenergetics and Biophysics at the Graduate School of Materials Science of Nara Institute of Science and Technology (Akira Isogai, President); Graduate School of Life Science (Yoshiki Higuchi, Professor, also a visiting scientist at RIKEN) of the University of Hyogo (Masayoshi Kiyohara, President); RIKEN SPring-8 Center (Tetsuya Ishikawa, Director) of RIKEN (Ryoji Noyori, President); Faculty of Pharmaceutical Sciences of Doshisha Women's College of Liberal Arts; and China West Normal University. Serpinopathies,*2 which may induce premature senility, hepatic cirrhosis, pulmonary emphysema, and thrombosis, are considered to be caused by the accumulation of chain-shaped unnecessary polymerized proteins in living tissues. This achievement is expected to clarify the causes and prevention of diseases induced by mutation in protein structure. Protein cyt c is present in mitochondria, which are organelles involved in cell respiration. For nearly half a century, it has been known that cyt c is an indispensable protein for respiration and forms polymers by extending its structure in a chain shape upon denaturation. However, the denaturation mechanism remains unknown. Professor Hirota and his colleagues synthesized cyt c polymers, specifically succeeded in synthesizing pure uniform polymers with crystal structures of dimeric, trimeric, and tetrameric cyt c, and analyzed these molecular structures their characteristics. Meanwhile, Professor Higuchi and his colleagues succeeded in crystallizing each structure and analyzed these molecular structures using synchrotron radiation X-rays at SPring-8. As a result, they found that domain swapping,*3 in which corresponding domains are swapped when two molecules come into contact, occurs during polymerization of cyt c, assuming that one molecule (monomer) is a structural unit consisting of several domains. The mechanism by which cyt c forms polymer chains by successive domain swapping in multiple molecules was clarified. The results of this research were published online in the American scientific journal Proceedings of the National Academy of Sciences (PNAS) on 6 July 2010. In proteins, various types of amino acid are sequenced in a long chain, and they form a specific steric structure depending on the sequence of the amino acids and exhibit particular functions. However, when proteins are structurally denatured, i.e., when the long chain of amino acids is not properly refolded or proteins are not properly decomposed to be removed from the cell, the proteins that lost their original functions due to the structure denaturation accumulate in living tissues, inducing diseases. The denaturation mechanism of cyt c clarified in this study is very similar to that proposed for serpinopathies. This achievement is expected to clarify the denaturation mechanism of protein, which is closely related to diseases. Publication: |
<Figure>
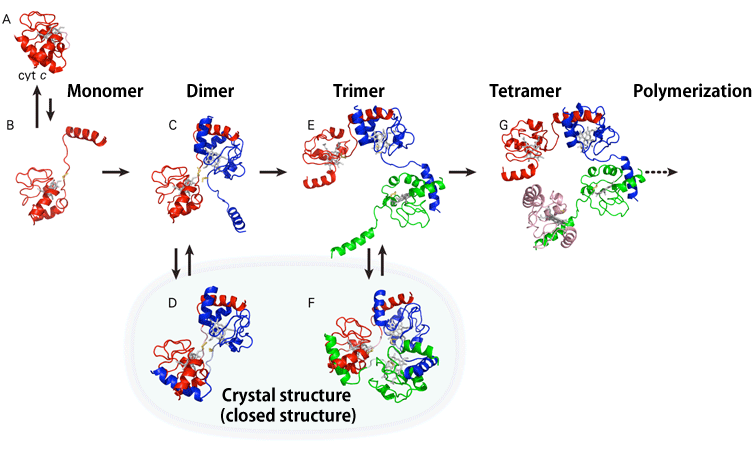
A: Crystal structure of cyt c monomer known thus far
B,C,E,G: Schematic of the process by which the cyt c monomer forms chains of dimeric, trimeric, and tetrameric cyt c
D,F: Crystal structures of dimeric and trimeric cyt c clarified in this study
<Glossary>
*1 Polymer
Polymers are compounds consisting of repeating structures of fundamental units (monomers) and are frequently referred to as compounds that are artificially polymerized. The repeating structures of biomolecules, such as proteins, are called biopolymers. Low-molecular-weight polymers, such as dimeric and trimeric complexes, are sometimes called oligomers, and are distinguished from polymers.
*2 Serpinopathies and conformational (folding) diseases
Serpin is a generic term for serine protease inhibitor proteins. Serpinopathies are a group of diseases induced by the polymerization of serpin; premature senility caused by mutant neuroserpin, hepatic cirrhosis, and pulmonary emphysema caused by α1 antitrypsin mutant, and thrombosis caused by antithrombin are well known. Serpinopathies are a type of conformational disease, which is a generic term for diseases caused by the formation of protein aggregates following the conformational change of proteins and their accumulation in cellular tissues. Alzheimer's disease, Parkinson's disease, and bovine spongiform encephalopathy (mad cow disease) are also considered as conformational diseases.
*3 Domain swapping
Domain swapping refers to the replacement of the secondary structure (α helix, β strand, looped domain) or tertiary structure (domain) in the dimeric and trimeric complexes consisting of the same types of protein molecule with the corresponding structure in another complex. In some cases, swapping occurs between structures that are clearly confirmed as domains; in other cases, only a loop invades into the other complex without having a clear domain structure.
For more information, please contact: Prof. Yoshiki Higuchi (University of Hyogo) |
- Current article
- Clarification of How Cytochrome c, an Important Protein Involved in Respiration, Loses Its Function by Joining in Chains, Half a Century after the Discovery of Its polymerization (Press Release)