Successful Analysis of Crystal Structure of Human Ero1 Promoting Steric Structure Formation of Proteins (Press Release)
- Release Date
- 11 Sep, 2010
- BL44XU (Macromolecular Assemblies)
- Facilitates understanding of the protein quality control mechanism in human cells and is expected to clarify pathogenesis of diseases such as neurodegenerative diseases, immunodeficiency, and diabetes -
Kyushu University
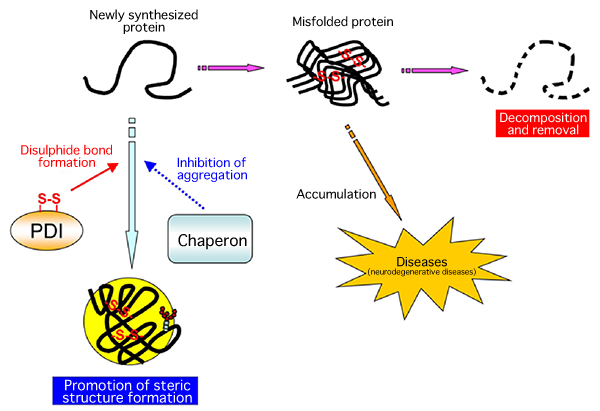
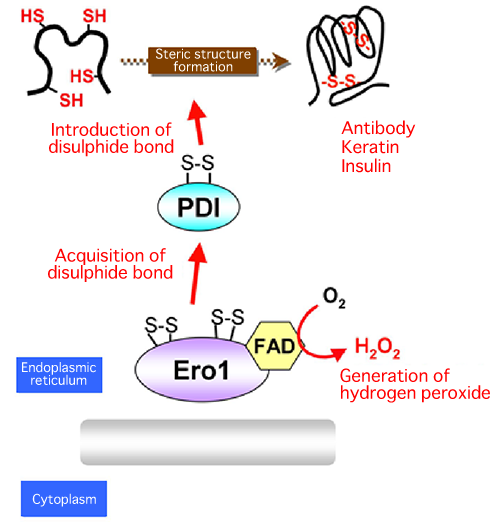
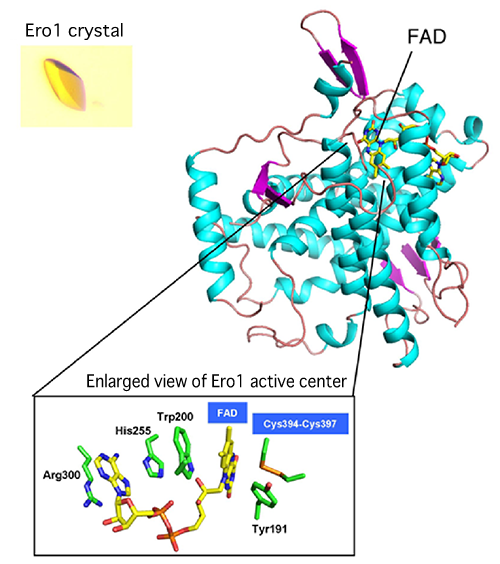
A joint research team consisting of scientists from Kyushu University, Osaka University, and Universita Vita-Salute San Raffaele Scientific Institute (team leader: Kenji Inaba, specially appointed associate professor of Medical Institute of Bioregulation, Kyushu University) succeeded in the high-resolution crystallography of Ero1, an enzyme that forms protein disulphide bonds in human cells. Thus far, research on protein disulphide bond formation factors in microorganisms, such as Escherichia coli and yeast, has been carried out; however, this is the first time in the world that the molecular structures and reaction mechanisms of these factors in human cells have been clarified. The findings revealed part of the steric structure formation mechanism of proteins in human cells as well as the quality control mechanism of proteins in the cells. In addition, the following has been clarified at the molecular level; Ero1, which generates reactive oxygen species (hydrogen peroxide) that cause aging, arteriosclerosis, and cancer, has a self-regulatory mechanism to control its own activities to prevent the generation of excess reactive oxygen species. These achievements are expected to lead to the clarification of the pathogenesis of diseases such as neurodegenerative diseases, immunodeficiency, and diabetes in the future. These research achievements were published online in a European scientific journal, The EMBO Journal, published by the Nature Publishing Group on 6 September 2010. (Publication) |
<Figure>
|
For more information, please contact: |
- Current article
- Successful Analysis of Crystal Structure of Human Ero1 Promoting Steric Structure Formation of Proteins (Press Release)