World’s First Clarification of Mechanism of Protein Export Enhanced by Effects of Ions (Press Release )
- Release Date
- 12 May, 2011
- BL41XU (Structural Biology I)
The University of Tokyo
Kyoto Sangyo University
Kyoto University
|
A group of researchers from the University of Tokyo, Kyoto Sangyo University, and Kyoto University (represented by Professor Osamu Nureki of the Graduate School of Science, the University of Tokyo, and Professor Koreaki Ito of the Faculty of Science, Kyoto Sangyo University) clarified, for the first time in the world, the detailed structure of a membrane protein, SecDF, which plays a significant role in the transmembrane transport of proteins synthesized by ribosomes in a cell. From the structural observation of SecDF, they proposed a hypothesis that “SecDF repeatedly undergoes major structural changes that are induced by the extent of the difference between the cation concentrations outside and inside the cell membrane and thus participates in the transmembrane transport of proteins.” They demonstrated this hypothesis using some biochemical and biophysical techniques. Their achievements obtained by the atomic-level analysis of protein transport, which is an essential and elemental phenomenon of life, will have a considerable impact on research in this field as well as research on the transmembrane transport of ions and medical agents into and out of cells. Their achievements were published online in the British scientific journal Nature on 12 May 2011. Publication: |
<<Figures>>
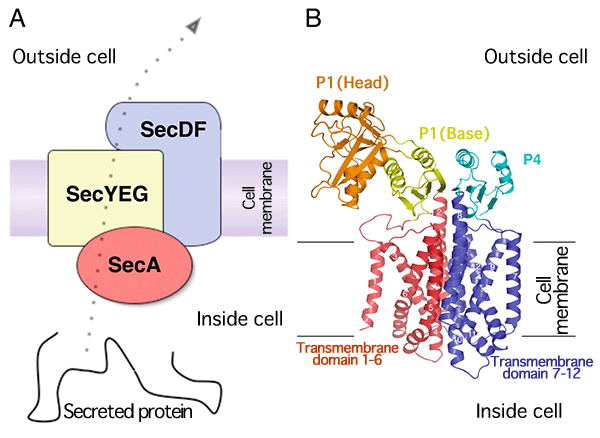
A:Schematic of transmembrane transport of protein mediated by Sec protein complex.
B:Crystal structure of SecDF. It consists of twelve transmembrane domains indicated in numbers (domains 1-6 and domains 7-12 are arranged in pseudo-twofold symmetry) and the domains protruding outside the cell (periplasm).
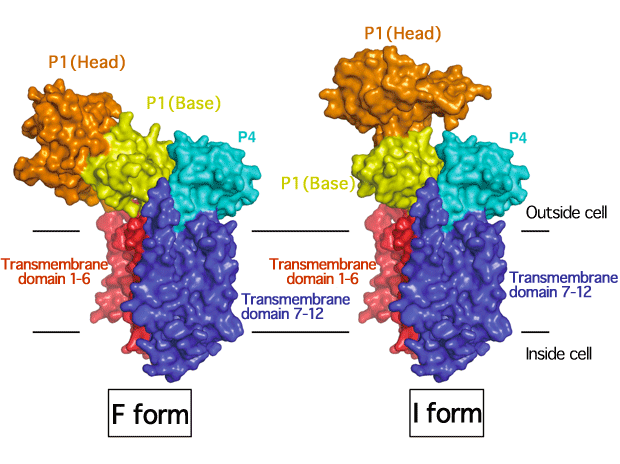
SecDF shows two different orientation patterns of the head of the P1 domain.
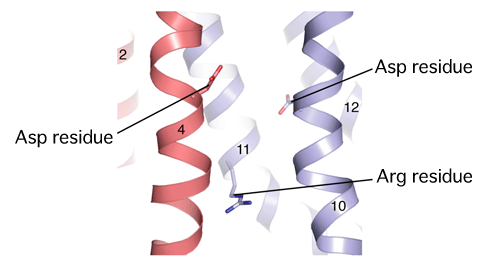
The figure shows an enlarged image of the center of the transmembrane domains. The numbers indicate the transmembrane domain number.
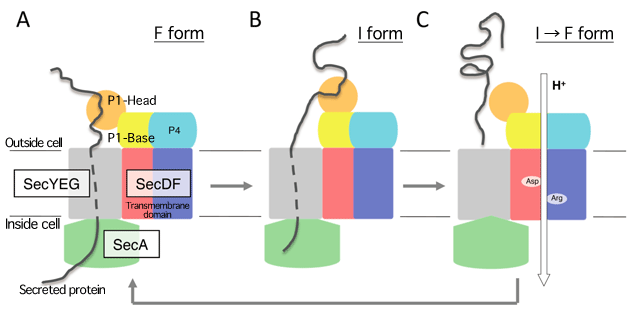
SecDF and SecYEG form a complex. The precursor protein, which is transported by SecA ATPase through SecYEG, interacts with the extracellular domain of SecDF outside the cell (A). Then, while holding the precursor protein, SecDF undergoes a structural change from the F form to the I form and allows the transport of the protein across the membrane (B). Finally, SecDF releases the protein when a proton is transported across the membrane, and it undergoes a structural change from the I form to the F form (C). The researchers proposed a model in which SecDF participates in the transmembrane transport of proteins through these processes.
*Essential arginine residues (Arg) and asparatic acid residues (Asp) are schematically shown in the figure.
|
For more information, please contact: Prof. Koreaki Ito (Kyoto Sangyo University) |
- Current article
- World’s First Clarification of Mechanism of Protein Export Enhanced by Effects of Ions (Press Release )