Research Results Strongly Suggest the Feasibility of a Sodium Ion Secondary Battery Containing No Rare Metals (Press Release)
- Release Date
- 30 Apr, 2012
- BL02B2 (Powder Diffraction)
Tokyo University of Science, Technology Licensing Organization (Accredited TLO)
|
A research group at Tokyo University of Science has successfully synthesized a novel Fe-based, layered oxide containing no rare metals for use in sodium-ion battery electrodes. The research was led by Dr. Naoaki Yabuuchi (lecturer, Research Institute for Science and Technology, Tokyo University of Science) and Dr. Shinichi Komaba (Associate professor, Department of Applied Chemistry, Faculty of Science Division I, Tokyo University of Science). The research accomplishments were published online in “Nature Materials” (http://www.nature.com/nmat/). The research group, led by associate professor Komaba, has been undertaking basic research, starting from 2005, on alternative materials for electric energy storage, namely, the use of a naturally abundant element (i.e. sodium) in place of lithium, which has gained widespread use in high-performance batteries. The group has successfully established the feasibility of a new lithium-free energy device (sodium-ion battery) that operates at room temperatures, whereby a carbon material and layered oxides are used. This research accomplishment was published in “Advanced Energy Materials” (a German scientific journal) and gained great attention around the world because of its implication for future power source technologies: the sodium-ion battery can be a future candidate for large fixed batteries deployed in the smart grid, as well as an alternative power source for electric vehicles. This time, the research was extended to a successful synthesis of novel, Fe-based layered oxides that use a combination of naturally abundant elements (Fe, Mn, and Na), and completely eliminates the need to use rare metals. It also demonstrated the possibility of achieving higher battery energy density as compared with conventional materials. This was a joint effort among the following researchers and an enterprise: Dr. Naoaki Yabuuchi (lecturer, Research Institute for Science and Technology, Tokyo University of Science), Associate professor Shinichi Komaba (Department of Applied Chemistry, Faculty of Science Division I, Tokyo University of Science), Professor Yasuhiro Yamada (Department of Chemistry, Faculty of Science Division II, Tokyo University of Science) and GS Yuasa Corporation (headquarters: Minami-ku, Kyoto; president: Mr. Makoto Yoda). These research results demonstrated, ahead of other countries, the possibility of constructing a high energy-density power storage device eliminating the need for such rare metals as lithium, cobalt, and nickel A part of this research (research leader: Shinichi Komaba) was supported by grant-in-aid from Japan Society for the Promotion of Science (“Funding Program for Next Generation World-Leading Researchers”*1). Some of the experiments were conducted using the facilities (beam line: BL02B2) at SPring-8 and the Photon Factory (beam line: BL-7C) at the Institute of Materials Structure Science (High Energy Accelerator Research Organization, KEK). Publication: |
<<Figures>>
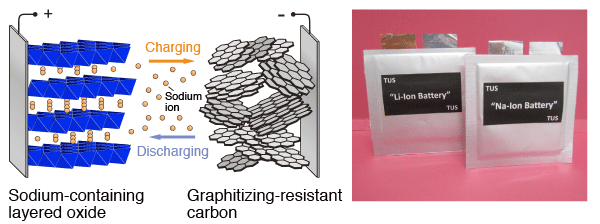
(Left) Diagram representing the principle of operation of a sodium ion cell. (Right) Photographs of a laminated lithium ion battery and a sodium ion battery (prototype)
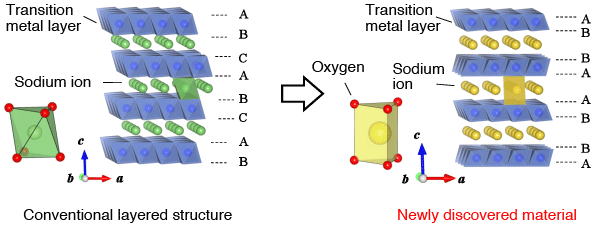
Comparison of conventional layered oxide (left) and the newly discovered Fe-based layered material. One of the key differences lies in the oxygen coordination environment in relation to sodium ions.
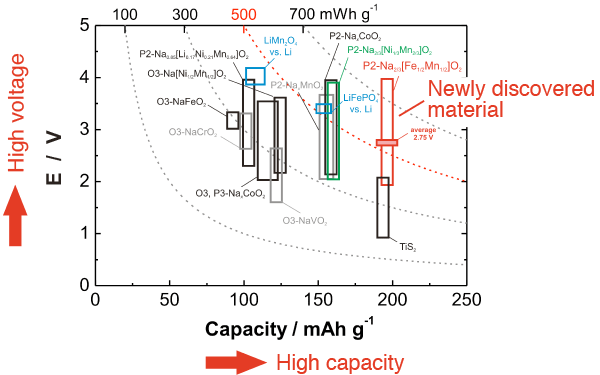
Energy density comparison among various sodium-containing anode materials
<<Glossary>>
*1 Funding Program for Next Generation World-Leading Researchers
This program provides a research-support system for researchers expected to have the potential to be world leaders in their respective fields of science and technology. The Japanese government's "New Growth Strategy (Basic Policies) Toward a Radiant Japan" (Cabinet decision on December 30, 2009) calls for advancing a wide spectrum of research from basic research that generates new sciences and technologies to R&D with near-future applications. By supporting the kind of cutting-edge research mandated by the New Growth Strategy, the program seeks to spur mid- to long-term S&T advancement, while contributing to the continued growth of Japan as a nation and the solution of policy-focused and societal issues. (an excerpt from the application guideline for grant-in-aid from Japan Society for the Promotion of Science)
|
For more information, please contact:
Dr. Naoaki Yabuuchi (Tokyo University of Science) |
- Previous Article
- Nanocrystalization brings about a breakthrough upgrade of electric conductivity in polymer films (Press Release)
- Current article
- Research Results Strongly Suggest the Feasibility of a Sodium Ion Secondary Battery Containing No Rare Metals (Press Release)