Nanocrystalization brings about a breakthrough upgrade of electric conductivity in polymer films (Press Release)
- Release Date
- 27 Apr, 2012
- BL40B2 (Structural Biology II)
Japan Synchrotron Radiation Research Institute
Tohoku University
University of Yamanashi
|
Researchers at Japan Synchrotron Radiation Research Institute (JASRI), Tohoku University, and University of Yamanashi discovered, for the first time in the world, evidence of nano-size crystalization of PEDOT molecules in electric conductive PEDOT:PSS*1 film, which has received widespread attention as the next generation organic electronic material. This discovery clearly indicates that the high electric conductivity originates from a hierarchical structure*2 consisting of types of nanocrystaline polymeric elements, and the information is indicative of new guidelines for the research and development of electric conductive polymer films. Because of the inherent characteristics of the conductive polymer films—lightweight and foldable—they have great potential to provide materials for displays and electronic device elements. In addition, they are capable of being produced using printing technology, contributing to a substantial reduction in the amount of materials and power consumption in the manufacturing processes. PEDOT:PSS has been widely used as a representative electric conductive polymer, but the structure and properties of the complex consisting of PEDOT and PSS molecules have been unclear. The selection of film-manufacturing procedures is known to exert a huge effect—an irregular magnitude of electric conductivity up to 1,000 times—, constituting an obstacle for reliable applications in high performance devices. The research group conducted structural analysis on these PEDOT:PSS films using the high-intensity, highly directional radiation X-ray beam available at SPring-8*3. The samples included those PEDOT:PSS films that exhibited huge electric conductivity variations depending on the manufacturing method used. The analysis made it clear for the first time in the world that, largely different from the presumptions up to now, PEDOT:PSS had a nanoscale core-shell structure in which a crystallized PEDOT molecule constituted a nanoscale core surrounded by a shell consisting of PSS molecules. It also demonstrated that an improved film manufacturing method may enlarge the size of the PEDOT nanocrystals and lead to a more orderly molecular arrangement within the core, resulting in a dramatic increase in electronic conductivity. The results of the research are considered to provide a guideline for material design and improving manufacturing processes for producing highly electric conductive PEDOT:PSS, even comparable to metallic materials in terms of electric conductivity, promoting breakthroughs in the developing efforts for electric conductive polymers. The results reported here were obtained through the research conducted by Dr. Akihiko Fujiwara (principal researcher, JASRI), Prof. Takahiko Sasaki (Institute for Materials Research, Tohoku University), Prof. Hidenori Okuzaki (University of Yamanashi) and their teams. The research results were published in the online publication of “Macromolecules,” an American Chemical Society journal dedicated to macromolecule science, on April 26, 2012 (US time). Publication: |
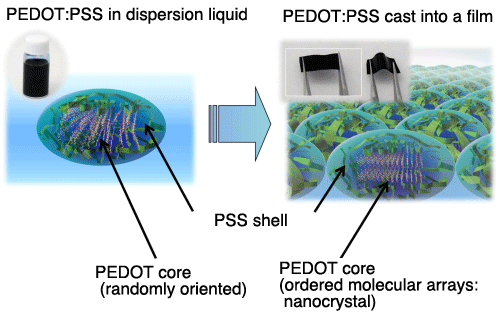
<<Figures>>
and the structure model of PEDOT:PSS film (right)
This is the first experimental confirmation of the core-shell structure consisting of PEDOT cores and PSS shells. PEDOT molecules are randomly oriented inside the core when dispersed in a liquid, and they tend to form a more orderly arrangement when cast into a film. The experimental results indicated that the formation of a nanocrystal, which is an orderly arrangement of PEDOT molecules within the entire core, gives rise to a dramatic increase in the electric conductivity of PEDTO:PSS.
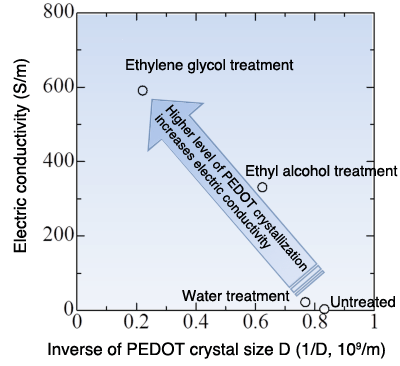
Immersing a film specimen in a polar molecule*5 solution contributes to enhanced PEDOT crystallization: the inverse of crystal size decreases. The clear relationship between the level of crystallization and conductivity— enhanced crystallization producing a dramatic increase in conductivity—was demonstrated for the first time in the world.
<<Glossary>>
*1 Conductive polymer material PEDOT:PSS
PEDOT:PSS is a polymer mixture in which polyethylenedioxythiophene (PEDOT) is added to polystyrene sulfonate (PSS). Because of its stability in air and good optical transparency, the polymer is commonly used as an antistatic coating for plastic materials. Expectations are rising for its wider applications in the future as a transparent electrode material without the need for rare metals (typically indium), in such products as organic EL displays and touch panels. A famous example of an electric conductive polymer includes polyacetyrene (Dr. H. Shirakawa was awarded with the Nobel Prize for discovering it), and PEDOT was developed to provide better electric conductivity. Although PEDOT has the structural characteristics to develop electric conductivity, it lacks carriers to transport electricity, thus PSS is added to provide an electric carrier to PEDOT.
*2 Hierarchical structure
A system organization in which an assembly consisting of multiple basic elements becomes, in turn, a constituting element of even greater regularity. In the case of PEDOT:PSS, covalently bonded atoms constitute a PEDOT and PSS molecule, and the molecules of the same species get together to form a cluster, crystal, and amorphous agglomeration, and finally these agglomerated bodies form a complex.
*3 SPring-8
A RIKEN facility located in Harima Science Garden City (Hyogo prefecture) is capable of producing the world's highest intensity synchronous radiation. The management and promotion of utilization of this facility are undertaken by JASRI. The name “SPring-8” comes from “Super Photon ring-8GeV.” An electron flying at nearly the speed of light, if deflected from its original trajectory through the effect exerted by a magnet, emits an electromagnetic wave in a direction tangential to its trajectory, which is called radiation light (or synchrotron radiation). At present, there are three “3rd Generation” large scale synchronous radiation facilities in the world: SPring-8 (Japan), APS (USA) and ESRF (France). The acceleration energy available at SPring-8 (8 billion electron volts) enables the provision of an extremely wide spectrum of radiation light: from far infrared to visible, vacuum ultraviolet, and soft X-ray up to hard X-ray. SPring-8 provides a theater for collaborative works involving researchers inside and outside Japan, and the research conducted at this facility cover such diverse areas as material science, geoscience, life science, environmental science, and various applications in industrial sectors.
*4 Micelle
A spherical assembly of molecules (or agglomerated molecules) characterized by possessing both hydrophilic (i.e. having an affinity for water) and hydrophobic (i.e. lacking an affinity for water) portion at their ends. The molecules (or agglomerated molecules) form a spherical structure in an aqueous solution with the hydrophobic ends oriented towards the center. Micelles are easily dispersed in an aqueous media and behave as a uniform solution (in the strict sense, they are not dissolved).
*5 Polar molecule
A molecule in which the center of positive charge does not coincide with that of negative charge. The non-coincidence of the centers of positive and negative charge distributions renders the molecule to have spontaneous and permanent dipole moments. Polar molecules include such common molecules as water, ethyl alcohol, and ethylene alcohol. Differences in the levels of electronegativity—the tendency of an atom to attract electrons—among the atoms in a molecule are the main driving force to produce a polar molecule.
|
For more information, please contact: Prof. Takahiko Sasaki (Tohoku University) Dr. Hidenori Okuzaki (University of Yamanashi) |
- Current article
- Nanocrystalization brings about a breakthrough upgrade of electric conductivity in polymer films (Press Release)