Rare-Earth Metal Hydride with Rock-Salt (NaCl) Lattice Structure Found (Press Release)
- Release Date
- 07 May, 2012
- BL22XU (JAEA Quantum Structural Science)
Japan Atomic Energy Agency
High Energy Accelerator Research Organization (KEK)
J-PARC Center
Hiroshima University
Synopsis
• The crystal structure of a novel hydride—a product of high-pressure decomposition of lanthanum di-hydride (LaH2)—was successfully elucidated through complementary use of a neutron beam (J-PARC) and a synchrotron radiation X-ray (SPring-8).
• Two powerful techniques were used to complement each other: a synchrotron radiation X-ray for analyzing metal lattice structure, and a neutron beam for metal and hydrogen structure. The approach proved effective in discovering the formation of a mono-hydrate (LaH) with a rock-salt (NaCl) lattice structure, leading further to confirmation that only the rare-earth metallic elements, among all metallic elements, can incorporate hydrogen atoms in three distinct forms.
• The findings encourage expectation for the clarification of the mechanism behind hydrogen-metal interactions, and for accelerated development of high-performance hydrogen storage materials.
|
The research group at Japan Atomic Energy Agency (JAEA, Director: Atsuyuki Suzuki) conducted research, in collaboration with High Energy Accelerator Research Organization (KEK, Director: Atsuto Suzuki), J-PARC Center (a research facility jointly operated by JAEA and KEK, Deputy center director: Masaji Nagamiya), Hiroshima University (president: Toshimasa Asahara), University of Tokyo (president: Junichi Hamada) and University of Cambridge (UK), on rare-earth metal*1 hydrides, and successfully elucidated the crystal structure using the high-intensity neutron beam available at J-PARC*2 and the synchrotron radiation X-ray available at SPring-8.*3 The findings include the world’s first observation of a yet unknown rare-earth mono-hydride (LaH) with a rock-salt (NaCl) lattice structure. Rare-earth metals (scarce metals) are known to have an excellent affinity to hydrogen, and often form a hydride (a metal-hydrogen compound that can contain a large amount of hydrogen). The hydrogen atom incorporated in a hydride occupies an interstice surrounded by metal atoms in one of the following two modes: a tetrahedral structure or an octahedral structure (in either case, the apex of the structure is occupied by metal atoms). Hydrogen atoms start occupying the interstices of a tetrahedral structure to form a bi-hydride, and then proceed to occupy those in an octahedral structure until total saturation (tri-hydride). Mono-hydride, where only the interstices in an octahedral structure are occupied by hydrogen atoms, is well known among transition metals,*4 but has not been reported yet among rare-earth metals. Prior to this study, the research group found that the di-hydride of lanthanum (LaH2), a typical rare-earth element, existed in two distinct states, i.e. hydrogen-rich and hydrogen-poor states. This time, a series of extreme high-pressure neutron diffraction experiments were carried out at the Materials and Life Science Experimental Facility (a facility in Japan Proton Accelerator Research Complex (J-PARC)). The experiments targeted the deuterium-substituted di-hydride (LaD2), leading to the first observation ever of the formation of two types of hydrides: a hydrogen-rich hydride that has a similar composition to tri-hydride (LaD3), and a hydrogen-poor counterpart with a composition similar to mono-hydride (LaD). LaD takes the rock-salt (NaCl) structure, with its interstice in an octahedral structure occupied by a deuterium atom. The stable existence of mono-hydride (LaH/LaD) under high pressure was also confirmed based on a first principle calculation.*5 The findings from this research demonstrated that only the rare-earth metals, among all metallic elements, can form three types of hydrides (mono-, di-, and tri-hydride) and all of their metallic lattices constitute a face-centered cubic structure. Owing to their excellent affinity to hydrogen, the rare-earth metals are widely used as a constituent element of hydrogen storage materials. A deeper understanding of the mechanism behind the interaction between hydrogen and metallic elements in the future, through detailed studies on hydrogen-metal binding in hydrides, holds promise with regard to helping set guidelines for developing rare-earth alloys capable of storing a large amount of hydrogen. The research results reported here were obtained through collaboration among the researchers and institutes listed below: The results of the research were issued in the online publication of “Physical Review Letters” (a US scientific journal) on May 8. Publication: |
<<Figures>>
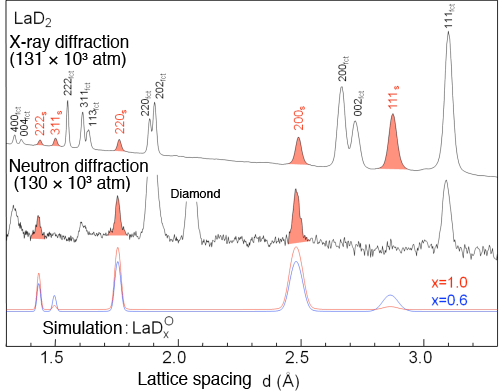
and a neutron taken under extreme high pressure (130 × 103 atm.)
The red profile represents the diffraction pattern from LaD. In the neutron diffraction pattern, lines ascribed to odd index number diffraction are not observed, clearly indicating the formation of a rock-salt structure. A result from diffraction pattern simulations carried out with varied occupancy ratios of deuterium (DO) on octahedral sites shows that the reproducibility of the experimental results became poor as the occupancy ratio decreased.
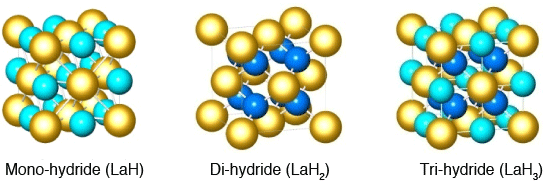
in a face-centered metallic lattice.
The yellow spheres represent a metallic atom, the light-blue spheres represent a hydrogen atom in an octahedral site, and the darker blue ones a hydrogen atom in a tetrahedral site. From left to right: mono-hydride (hydrogen atoms in octahedral sites), di-hydride (hydrogen atoms in tetrahedral sites), and tri-hydride (hydrogen atoms in both sites). The type of hydrides with hydrogen atoms in octahedral sites constitutes a typical rock-salt (NaCl) structure; this is the first instance where the formation of a mono-hydride with a rare-earth metal base is observed.
<<Glossary>>
*1 Rare-earth metal
The term generically refers to the following 17 elements of the periodic table including the lanthanoid group (atomic numbers 57 (La) to 71 (Lu), 15 elements in all), Scandium (Sc; atomic number 21), and Yttrium (Y; atomic number 39). They have important industrial applications as magnetic materials, in addition to use for metal alloys for hydrogen storage.
*2 Japan Proton Accelerator Research Complex (J-PARC)
An experimental facility complex (Tokai village, Ibaraki pref.) constructed as a joint project between Japan Atomic Energy Agency and KEK, which includes one of the world’s most powerful proton accelerator facility and experimental facilities. The acronym “J-PARC” stands for Japan Proton Accelerator Research Complex. J-PARC provides experiment opportunities to cutting-edge academic studies and industrial applications in such wide areas as material science, life science, atomic nucleus, and elementary particle physics. The main tools for these experiments are the secondary particles (neutrons, muons, mesons, and neutrinos) generated by bombarding nuclear targets with accelerated protons. Neutron-related research is carried out in the Materials and Life Science Experimental Facility (a J-PARC facility), in which one of the world’s most powerful pulsed neutron beams is available.
*3 SPring-8
A RIKEN facility located in Harima Science Garden City (Hyogo prefecture) is capable of producing the world's highest intensity synchronous radiation. The management and promotion of utilization of this facility are undertaken by JASRI. The name “SPring-8” comes from “Super Photon ring-8GeV.” An electron flying at nearly the speed of light, if deflected from its original trajectory through the effect exerted by a magnet, emits an electromagnetic wave in a direction tangential to its trajectory, which is called radiation light (or synchrotron radiation). At present, there are three “3rd Generation” large scale synchronous radiation facilities in the world: SPring-8 (Japan), APS (USA) and ESRF (France). The acceleration energy available at SPring-8 (8 billion electron volts) enables the provision of an extremely wide spectrum of radiation light: from far infrared to visible, vacuum ultraviolet, and soft X-ray up to hard X-ray. SPring-8 provides a theater for collaborative works involving researchers inside and outside Japan, and the research conducted at this facility cover such diverse areas as material science, geoscience, life science, environmental science, and various applications in industrial sectors.
*4 Transition metals
The term generically refers to the elements in the periodic table that belong to the third to eleventh groups.
*5 First-principle calculation
The term refers to theoretical calculations performed without resorting to empirical parameters and experimental data. For example, material properties, such as crystal structure and electronic state, are calculated from the interactions between the nucleus and electrons based on quantum mechanical principles.
|
For more information, please contact: Prof. Toshiya Otomo (Institute of Materials Structure Science, KEK) |
- Previous Article
- New Disturbance-Resistant Quantum Liquid State Discovered in a Copper-Oxide Magnetic Body (Press Release)
- Current article
- Rare-Earth Metal Hydride with Rock-Salt (NaCl) Lattice Structure Found (Press Release)