The World’s First Nanoscale Observation of the Operating Interface of a Lithium Ion Battery (Press Release)
- Release Date
- 25 Oct, 2012
- BL01B1 (XAFS)
- BL37XU (Trace Element Analysis)
Kyoto University
|
The research group at Kyoto University conducted a study on the operating behavior of a lithium ion battery*2, and succeeded in the world’s first in-situ observation of the operating outermost surface of the electrode, elucidating the initial degradation process of the storage cell. The study is a part of the RISING project*1 (leader: Zenpachi Ogumi, specially appointed professor) jointly promoted by Kyoto University and the New Energy and Industrial Technology Development Organization (NEDO). The research group includes Dr. Daikou Takamatsu* (special-appointment fellow), Dr. Yukinori Koyama (special-appointment associate professor), Dr. Yuuki Orihara** (research fellow), Dr. Hajime Arai (special-appointment professor) and others. The research results were published in the online version of Angewandte Chemie International Edition (a German Chemical Society publication) on the 12th of October, 2012. * Office of society-academia collaboration for innovation, **Graduate school of human and environmental studies Publication: |
Background
Storage cells have been widely used in such applications as a source for mobile devices and for driving starter motors in automobiles. In recent years, an increasingly wide range of applications has been opening for them, such as electric automobiles and natural energy storage, and they are considered to be one of the key devices to address energy, environmental, and other challenges. Expectations are high for the performance enhancement of high-energy-density lithium ion batteries, especially for achieving better use-life characteristics to address long term use, typically in electric automobiles.
As one of the major factors leading to the degradation of a lithium ion battery, a reaction barrier is known to exist in the electrode-electrolyte interface, hindering the free passage of lithium ions. An effective measure to reduce the barrier is an integral part for future development, which requires in-situ observation of the interface while the battery is operating. The lack of practical techniques, however, has hindered nanoscale observation of the interface at work. The development of a proper analysis technique has strongly been desired.
Achievement
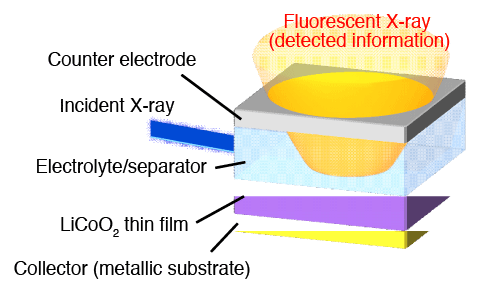
In this research, an experiment system is constructed in search of gaining information on the nanoscale behavior taking place at the interface during the charging/discharging process, wherein X-ray absorption spectroscopy (XAS)*4 is used to capture electronic behavior and localized structures of the material. High intensity synchrotron radiation available at SPring-8*3 was used as the X-ray source, enabling the gathering of focused information from the targeted interface among other cell elements. The focus of the research was placed on LiCoO2, a common material used in the positive electrode of lithium ion batteries, and a thin, flat membrane was prepared for the experiment for easier observation of the interface (Fig. 1).
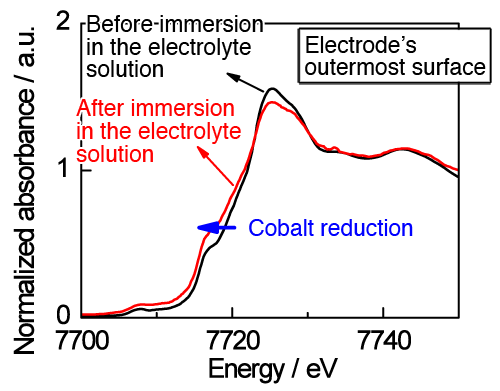
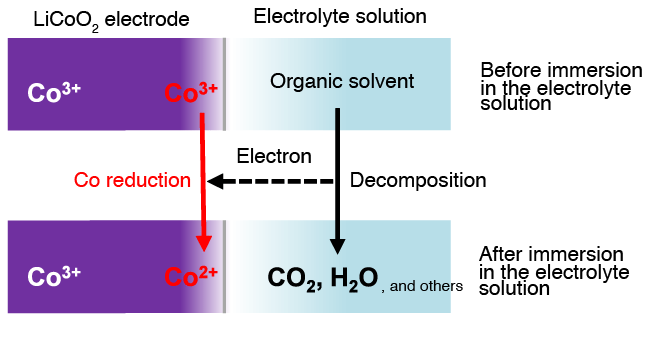
Comparison of XAS data taken before and after the immersion of LiCoO2 electrode in an electrolyte solution showed that the cobalt species were reduced at the wetted outermost surface of the electrode, while no change was observed inside (several tens of nanometers from the surface) (Fig. 2). When the system undergoes charging and discharging, well synchronized reversible reactions were observed inside the electrode. In contrast, irreversible behaviors were apparent at the outermost surface, indicating that the reduction of cobalt species, induced through contact with the electrolyte, can hinder the electrode reactions from proceeding smoothly. As the present knowledge did not expect the reduction of cobalt species to occur at the outermost surface, quantum mechanical calculations were carried out to evaluate energy relationships. The results of these theoretical calculations clearly indicate that the oxidation of an organic solvent and reduction of cobalt species can take place simultaneously at the outermost LiCoO2 electrode surface, under the action of the organic components contained in the electrolyte (Fig. 3).
Future prospects
The behavior of chemical species at the outermost surface of an electrode is important knowledge, but it has remained, up to now, beyond the reach of conventional macroscopic observation techniques and breaking-up approaches. Looking forward, the researchers are planning to extend the results and experience of this study for analyzing chemically modified surfaces and decomposition-inhibiting agents of electrolytes, leading to a longer life and enhanced performance of the lithium ion battery. Capitalizing on this knowledge, the researchers are also considering developing novel alternative storage batteries superior to the lithium ion battery in terms of performance.
<<Figures>>
at the outermost electrode induced by immersion in an electrolyte solution.
of the electrode, induced by the contact with electrolyte solution
<<Glossary>>
*1 RISING project
The Research and Development Initiative for Scientific Innovation of New Generation Batteries (RISING) is a joint research project promoted by the New Energy and Industrial Technology Development Organization (NEDO), aiming at developing novel storage batteries with 5-times as much energy density as those available today. RISING is a Japan-wide project involving 12 universities, 4 research organizations, and 13 enterprises, with the main research facilities located in Kyoto University and AIST (National Institute of Advanced Industrial Science and Technology) Kansai.
*2 Lithium-ion battery
A family of secondary cells widely used, owing to their high energy density, as the main source of a variety of hand-held electronics devices, such as mobile phones and notebook computers. In recent years, its application has been finding its way into such fields as hybrid/electric automobiles and large scale rechargeable batteries. Japan has had the largest share in the world for a long time, but is now facing tough competition with other countries, calling for attention to enhancing international competitiveness. The main constituents of this battery are the positive electrode, negative electrode, and electrolyte: lithium ions move back and forth during the charging and discharging process.
*3 SPring-8
A RIKEN facility located in Harima Science Garden City (Hyogo prefecture) is capable of producing the world's highest intensity synchronous radiation. The management and promotion of utilization of this facility are undertaken by JASRI. The name “SPring-8” comes from “Super Photon ring-8GeV.” An electron flying at nearly the speed of light, if deflected from its original trajectory through the effect exerted by a magnet, emits an electromagnetic wave in a direction tangential to its trajectory, which is called radiation light (or synchrotron radiation). At present, there are three “3rd Generation” large scale synchronous radiation facilities in the world: SPring-8 (Japan), APS (USA) and ESRF (France). The acceleration energy available at SPring-8 (8 billion electron volts) enables the provision of an extremely wide spectrum of radiation light: from far infrared to visible, vacuum ultraviolet, and soft X-ray up to hard X-ray. SPring-8 provides a theater for collaborative works involving researchers inside and outside Japan, and the research conducted at this facility cover such diverse areas as material science, geoscience, life science, environmental science, and various applications in industrial sectors.
*4 X-ray absorption spectroscopy (XAS)
X-ray absorption analysis (XAS) is an analytical technique for acquiring atomic scale information, such as the electronic structure of the atoms inside a substance and the local structure binding neighboring atoms. In this technique, the sample is irradiated by a high-energy X-ray beam, and the absorptions are measured at the energy levels associated with the target element.
|
For more information, please contact: |
- Previous Article
- Elucidation of the Origin of High Oxygen Permeability in Praseodymium-Nickel Oxides (Press Release)
- Current article
- The World’s First Nanoscale Observation of the Operating Interface of a Lithium Ion Battery (Press Release)