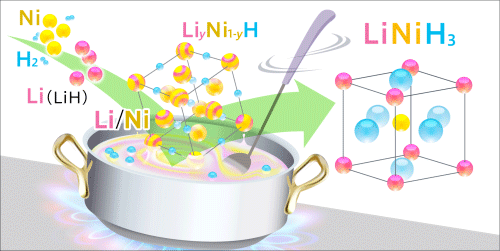
A new guideline for the development of novel functional hydrides: Elucidation of the formation process of perovskite-type hydrides (Press Release)
- Release Date
- 07 Mar, 2013
- BL14B1 (JAEA Materials Science)
Tohoku University
Japan Atomic Energy Agency (JAEA)
Key research findings
• A new hydride LiNiH3 with a perovskite structure was synthesized by hydrogenation under high-temperature and high-pressure conditions. The formation process was observed in situ by high-brilliance synchrotron X-ray diffraction to clarify the formation mechanism.
• In addition to their functions as hydrogen storage materials, perovskite-type hydrides are expected to show various physical properties and functions, such as superconductivity, similar to perovskite-type oxides. However, there have been few reports on the synthesis of perovskite-type hydrides.
• The clarification of the formation mechanism will provide guidelines for the design and development of perovskite-type hydrides, promoting the research and development of new materials.
|
A research group at the Institute for Materials Research (IMR; director, Mitsuo Niinomi), Tohoku University, in collaboration with the Advanced Institute for Materials Research (AIMR; director, Motoko Kotani), Tohoku University, and JAEA (president, Atsuyuki Suzuki), clarified the formation mechanism of a perovskite-type hydride using the high-brilliance synchrotron X-rays from SPring-8 for the first time. While perovskite-type hydrides are expected to be used as functional materials, there have been few reports on their synthesis. The guidelines for the design and development of the perovskite-type hydride determined in this study will promote the development of energy materials full of functions such as hydrogen storage and superconductivity. Perovskite structures*1 are well known in the form of oxides. Perovskite-type oxides with various physical properties and functions, such as superconductivity, ferroelectricity, and ionic conductivity, have been synthesized and utilized as practical materials such as piezoelectric devices. Similarly, perovskite-type hydrides,*2 in which oxygen atoms of perovskite-type oxides are replaced with hydrogen atoms, are expected to have various physical properties and functions in addition to hydrogen storage; however, few perovskite-type hydrides have been successfully synthesized thus far. Perovskite-type hydrides have been synthesized by mechanochemical processing,*3 the formation process of which remains unclear because it is difficult to observe the process. Hence the development of perovskite-type hydrides has been at a standstill. The research group succeeded in synthesizing a new perovskite-type hydride LiNiH3 based on the predictions obtained by first-principles calculations.*4 A powder mixture of lithium hydride (LiH) and nickel (Ni) was hydrogenated using high-temperature and high-pressure hydrogen fluid to synthesize a perovskite-type hydride with high crystallinity as a sintered body. The process of hydrogenation under high temperature and high pressure was observed by time-resolved X-ray diffraction*5 using the high-brilliance synchrotron X-rays to clarify the formation process of the perovskite-type hydride LiNiH3. The diffraction profiles showed that the perovskite-type hydride was formed not directly from the hydrogenation of LiH and Ni but through a three-step reaction: (I) the formation of nickel hydride (NiH), (II) the formation of a solid solution*6 of LiH and NiH (LiyNi1-yH), and (III) the formation of the perovskite-type hydride LiNiH3 induced by the absorption of hydrogen by the solid solution. Also, it was found that the formed solid solution, LiyNi1-yH, played the role of the precursor in the perovskite formation. The formation process clarified in this study suggests that choosing a combination of elements that can form solid-solution hydrides leads to the synthesis of perovskite-type hydrides, which can serve as guidelines for the design and development of perovskite-type hydrides. This achievement will become a breakthrough in the research on the synthesis of new perovskite-type hydrides. A part of this research was supported by the “Funding Program for Next Generation World-Leading Researchers” of the Cabinet Office under the title “Discovering Properties of Hydrides: from Basic Research on Hydrogen States to Energy-Device Demonstrations” and carried out as a research proposal at SPring-8. This achievement was published online in the American scientific journal Applied Physics Letters on 4 March 2013. |
<<Figures>>
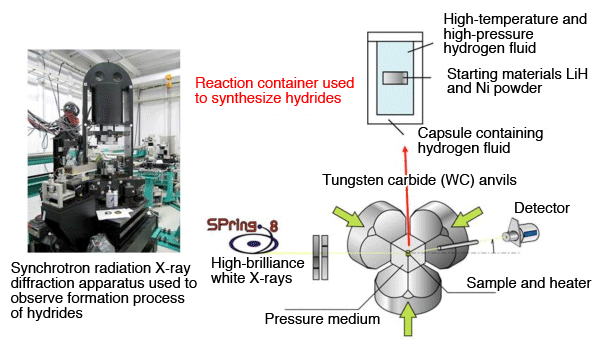
by high-brilliance synchrotron X-ray diffraction experiment
The process of the conversion of LiH and Ni powder to a perovskite-type hydride through hydrogenation was observed using high-brilliance synchrotron X-ray diffraction. The diffraction apparatus was installed at the beamline BL14B1 of SPring-8 by JAEA. The X-ray diffraction profiles of the formation process were measured every minute for 250 minutes until the formation of the perovskite-type hydride was completed. The analysis of those profiles led to the clarification of the formation mechanism.
of formation of perovskite-type hydride
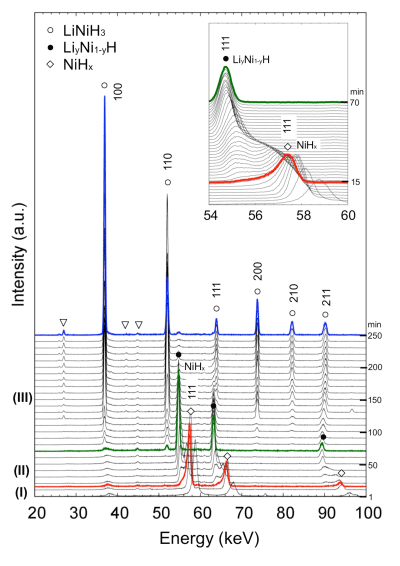
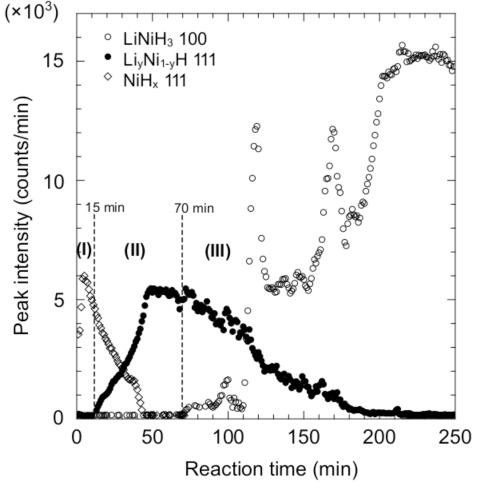
The representative profiles of the three steps described above, (I), (II), and (III), are shown by the red, green, and blue lines, respectively.
profiles shown by red, green, and blue lines in Fig. 1
The reaction proceeds from Step (I) to Step (II) to Step (III) with time.
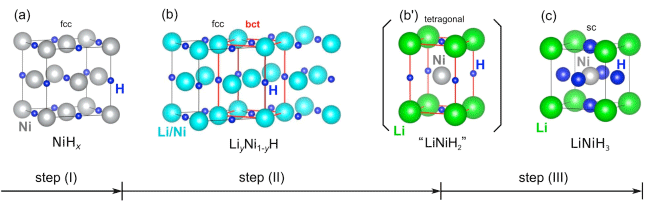
crystal structures obtained by analysis of X-ray diffraction profiles
At the metal lattice points in the solid solution LiyNi1-yH (b) formed in Step (II), Li and Ni atoms are present in the same proportion. In LiNiH3 (c) formed in Step (III), a Ni atom at the center of the cube is surrounded by eight Li atoms at the tetragonal corners and six H atoms at the centers of the faces of the cube. From these crystal structures, it is assumed that the solid solution (b) is converted to the perovskite-type hydride (c) through the intermediate (b’).
<<Glossary>>
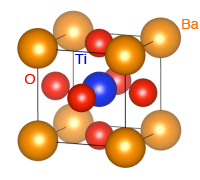 *1 Perovskite structures
*1 Perovskite structures
Chemical compounds represented by the chemical formula ABX3, in which A and B (cations of different metals) and X (an anion such as an oxygen atom) are ionically bonded to form a crystal. As shown by the crystal structure of a typical perovskite-type oxide, barium titanate (BaTiO3), the A metal cations (Ba2+, yellow) are located at the cubic corners, the B metal cations (Ti4+, blue) are located at the center of each cube, and the anions (O2-, red) occupy the face-centered positions.
*2 Perovskite-type hydrides
Hydrides with a perovskite-type crystal structure. X in the composition formula ABX3, which is generally oxygen (O), is replaced with hydrogen (H).
*3 Mechanochemical processing (ball milling)
Method of synthesizing nanoparticles by rotating a container containing metal balls and powder samples. Final products are synthesized by the repeated crushing and compacting of the samples caused by the collisions between the samples and the balls and container walls.
*4 First-principles calculations
Theoretical calculations performed without empirical parameters or experimental data. The material properties, such as crystal and electronic structures, are calculated from the interaction among nuclei and electrons on the basis of quantum mechanics.
*5 Time-resolved X-ray diffraction
An X-ray diffraction method to observe the structural changes in a material associated with reactions. An intense synchrotron X-ray source is required to obtain diffraction profiles at short intervals. In this study, X-ray diffraction profiles were measured every minute.
*6 Solid solution
A homogenous solid phase of a mixture of two or more elements. In this study, LiH and NiH with the same rock salt structure formed a solid solution, absorbed hydrogen, and formed a perovskite-type hydride LiNiH3.
|
For more information, please contact: |
- Current article
- A new guideline for the development of novel functional hydrides: Elucidation of the formation process of perovskite-type hydrides (Press Release)