World’s First Observation of Nonequilibrium Phase Transition of Positive Electrode Material for Lithium-Ion Batteries (Press Release)
- Release Date
- 10 Apr, 2013
- BL28XU (RISING)
Kyoto University
|
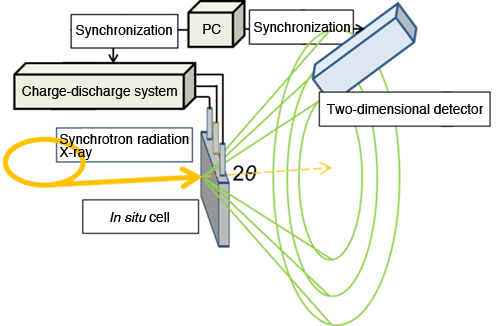
A research group of Kyoto University has clarified the transient state of the phase transition*3 of a positive electrode material used for lithium-ion batteries*2 and the mechanism behind a material that can be used for fast-charge and fast-discharge batteries. This study was part of the RISING Project*1 (project leader, Zempachi Ogumi**, professor) supported by Kyoto University and the New Energy and Industrial Technology Development Organization (NEDO). The group was led by Yuki Orikasa* (assistant professor), Yukinori Koyama** (associate professor), Katsutoshi Fukuda** (associate professor), Hajime Arai** (professor), and Yoshiharu Uchimoto* (professor). *Graduate School of Human and Environmental Studies The results of this research were posted online as a Just Accepted Manuscript in Journal of the American Chemical Society on 1 April 2013. Publication: |
<<Figures>>
under operating conditions of lithium-ion battery
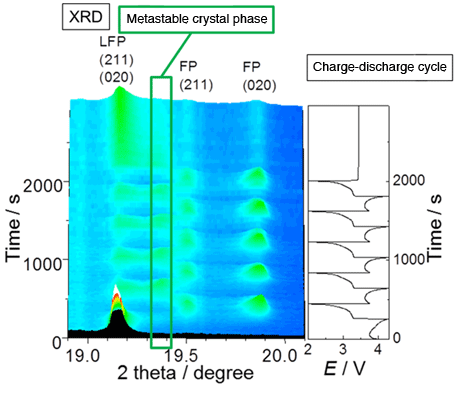
and fast-discharging reactions of LiFePO4
(The boxed diffraction pattern is not observed in the steady state and is only observed during the fast reaction.)
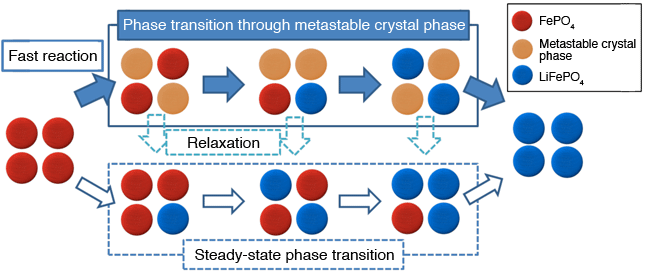
during fast reaction of LixFePO4
<<Glossary>>
*1 Research and Development Initiative for Scientific Innovation of New Generation Batteries (RISING) project
This project is based at and being carried out by Kyoto University and the National Institute of Advanced Industrial Science and Technology (AIST) Kansai in cooperation with various organizations throughout Japan (12 universities, 4 research institutes, and 13 companies). The aim of this project is to realize innovative rechargeable batteries with an energy density fivefold higher than that of currently available batteries. This project is one of the joint research projects supported by NEDO.
*2 Lithium-ion battery
Lithium-ion batteries are secondary (rechargeable) batteries with a high energy density and have been widely used as the main power sources of mobile electronic devices such as cell phones and laptop PCs. Lithium-ion batteries are mainly composed of a positive electrode, a negative electrode, and an organic electrolyte, and their charging and discharging are based on the movement of lithium ions. Recently, lithium-ion batteries have been applied to electric vehicles and large rechargeable batteries, intensifying the worldwide competition for their research and development.
*3 Phase transition
In lithium-ion batteries, lithium ions move between the positive and negative electrodes during charging and discharging. During these processes, the crystal structures of the positive and negative electrodes change. The phase of the positive electrode material examined in this study, LiFePO4, is separated into LiFePO4 and FePO4 phases during charging and discharging, and the reaction proceeds with changing their proportion. In this study, the phase transition behavior between LiFePO4 and FePO4 was analyzed.
|
For more information, please contact: |
- Previous Article
- Suppression of Amplification Errors during Polymerase Chain Reaction (PCR) (Press Release)
- Current article
- World’s First Observation of Nonequilibrium Phase Transition of Positive Electrode Material for Lithium-Ion Batteries (Press Release)