Visualization of Solvated Electrons in Large Cage Structure of Glass (Press Release)
- Release Date
- 28 May, 2013
- BL01B1 (XAFS)
- BL04B2 (High Energy X-ray Diffraction)
Japan Synchrotron Radiation Research Institute (JASRI)
Institute of Industrial Science, The University of Tokyo
Yamagata University
|
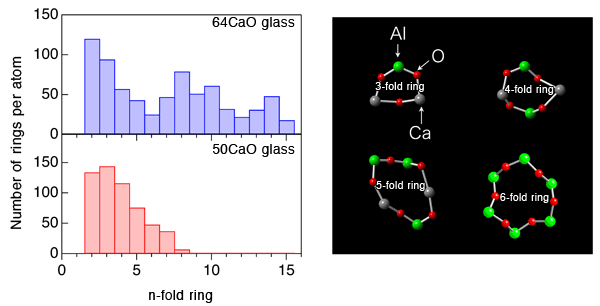
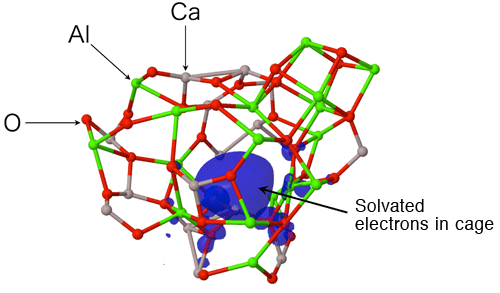
An international joint research team from JASRI, Tampere University of Technology, The University of Tokyo, Yamagata University, Osaka Prefecture University, Materials Development Inc., and Argonne National Laboratory clarified that the formation of large cage structure associated with the formation of large-sized rings in an oxide glass is the origin of its glass-forming ability. This outstanding finding was obtained through experiments using the high-brilliance synchrotron X-rays at SPring-8*1 and neutrons at Argonne National Laboratory, and by computer simulation using a supercomputer at Forschungszentrum Jülich GmbH (Jülich Research Center). The research team also clarified that glass structure is stabilized by solvated electrons in the cage of glass upon subtraction of some oxygen atoms from the glass. Glass is generally fabricated by cooling rapidly a high-temperature melt*2 of the raw materials. Raw materials however do not always form a glass; when the mixing ratio (composition) of the materials is changed, the materials may form crystals. To understand this phenomenon, the structural comparison of high glass-forming ability glass (high GFA glass) and low glass-forming ability glass (low GFA glass) gives important information. However, the atomic arrangement of glasses is, unfortunately, irregular, unlike that of crystals, and thus understanding the structure of glass has been considered to be difficult. The research team fabricated high GFA and low GFA calcium aluminate glasses having different compositions to compare and analyze their atomic arrangements by containerless processing (Fig. 1). The research team found, for the first time, that a large cage structure is formed in high GFA glass associated with the formation of large-sized rings (Fig. 2), whereas no cage structure is formed in low GFA glass. It has been reported that electride glass*3 can be fabricated from calcium aluminate melt. In this study, the research team demonstrated that solvated electrons*3 in the cage structure stabilized the glass structure energetically (Fig. 3) . The achievement of this study clarifies the relationship between the glass structure and the glass-forming ability, and solves one of the mysterious points in this field. Understanding the glass structure at the electronic level, as obtained in this study, will provide an important foundation for the development of innovative materials such as conductive glasses. This achievement was realized by the collaborative research team led by Dr. Shinji Kohara (Senior Scientist) of JASRI and was published online in the American scientific journal Proceedings of the National Academy of Sciences of the United States of America (PNAS) on 30 May 2013 (110 (25) 10129-10134). Publication: |
<<Figures>>
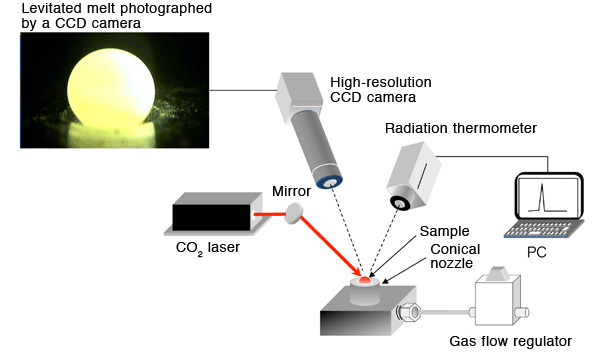
The sample is levitated by the gas ejected from a conical nozzle, heated and melted using a CO2 laser.
<<Glossary>>
*1 SPring-8
SPring-8 is a synchrotron radiation facility that provides the world’s highest-brilliance synchrotron radiation. It is owned by RIKEN and located in Harima Science Park City, Hyogo Prefecture, Japan. JASRI is responsible for the operation, management, and promotion of the use of SPring-8. The name “SPring-8” is derived from “Super Photon ring-8 GeV”. When the direction of the electron beams accelerated to nearly the speed of light is changed by magnets, electromagnetic waves are emitted in the tangential direction; these waves are synchrotron radiation. When the electron beam has a higher energy and the change in the traveling direction is large, synchrotron radiation contains shorter-wavelength lights such as X-rays. In particular, the following three facilities are known as the third-generation large synchrotron facilities: SPring-8 in Japan, the Advanced Photon Source (APS) in the USA, and the European Synchrotron Radiation Facility (ESRF) in France. Because the ring at SPring-8 enables an electron acceleration energy of 8 giga-electronvolts to be generated, synchrotron radiation in a wide range of wavelengths can be obtained including far-infrared light, visible light, vacuum ultraviolet light, soft X-rays, and hard X-rays. SPring-8 is used by researchers both in Japan and overseas for joint research in various fields such as materials science, earth science, life science, environmental science, and industrial applications.
*2 Melt
A melt is essentially the same as a liquid. Liquids of metals and oxides with a high melting point are frequently called melts.
*3 Electride glass and solvated electrons
Electride is originally an ionic compound in which an electron acts as an anion. For simplicity, the electrons acting as anions are assumed to solvate into a solid here. In-depth research on electrides has started with the investigation of ammonium solutions of alkali metals in which electrons are solvated. The electride of calcium aluminate was discovered in 2011 by Professor Hideo Hosono's group at Tokyo Institute of Technology. When some of the oxygen atoms are removed from calcium aluminate melt in a reducing atmosphere, an electride glass is obtained from the melt due to solvated electrons. This phenomenon attracts much attention because of its various functions such as coloring and high electrical conduction.
*4 Containerless processing (Fig. 1)
In containerless processing, a sample is levitated with zero gravity condition by inert gas (such as argon or nitrogen) blowing from a conical nozzle. The levitated sample is irradiated to melt by a laser that can easily realize an extremely high temperature exceeding 2,000°C. By using this method, contamination from the container can be prevented. In addition, liquid state (supercooled liquid state) may be maintained at rather lower temperatures than the real melting point because there are no boundaries between the melt and the container. The absence of boundary prevents the crystallization of the liquid. As a result, even glasses with low glass-forming ability can be fabricated.
|
For more information, please contact: Research Associate Atsunobu Masuno (Institute of Industrial Science, the University of Tokyo) Prof. Takeshi Usuki (YAMAGATA UNIVERSITY) |
- Current article
- Visualization of Solvated Electrons in Large Cage Structure of Glass (Press Release)